
Reducing Footprints and Increasing Output in Adherent Cell Culture
A Guest Blog by Jose Castillo, Director of Cell Culture Technologies at Pall LifeSciences
The cells used to make many biologic products sometimes require a surface on which the cells adhere as they grow. Many vaccines, for example, are made in this way. Historically, the surface has been provided either by a two-dimensional (2D) system such as roller bottles, T-flasks, cell factories or cell stacks; or microcarrier beads within a traditional stirred tank bioreactor.
Both methods have their advantages and disadvantages. Processes using the 2D systems are relatively straightforward to implement, and scale-up simply involves multiplying the number of devices being used. However, the inevitable impact on footprint involves significant space.
Microcarrier technology in a stirred bioreactor utilizes small beads of glass, plastic or other material that are added to suspension. The microcarriers provide a surface upon which adherent cells can attach and proliferate. The footprint of a microcarrier-stirred tank combination is much lower than 2D systems, but a successful process can be more difficult to develop.
An alternative to traditional 2D systems and to a microcarrier-stirred tank option comes in the form of the Integrity® iCELLis® system from Pall. The fixed-bed disposable bioreactor provides a more efficient and cheaper method for scaling up adherent cell cultures and it has a small footprint. In addition, labor and expertise requirements are much lower.
The System

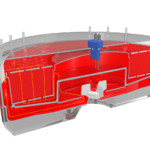
At its heart are multiple individual, nonwoven and hydrophilized macrocarriers made from polyethylene terephthalate. Each of these has a surface area of 11.2cm², [Image 1] and is compacted into a 25 liter fixed bed, shaped like a doughnut. These toroidal fixed-bed matrices are then incorporated within a bioreactor, with a working volume between 59 and 71 liters. An internal centrifuge pump within the bioreactor ensures a homogeneous distribution of media and cells throughout the fixed bed. In addition, a proprietary falling film system within the bioreactor ensures efficient gas transfer [Image 2].
The result is a fixed-bed system with an overall surface area of 500m² for the largest available unit, and significant opportunities for process intensification. This is equivalent [1] to nearly 6,000 standard roller bottles [Image 3], almost 800 of Thermo Scientific’s Cell Factory™ systems or Corning® CellSTACKs or a 650 liter stirred bioreactor containing 3g/l Cytodex 1 microcarriers from GE Healthcare.
Simplified Scale-up
One challenge for adherent cell-based manufacturing is time and resources necessary to scale-up up sufficient cell mass required for large-scale manufacturing. Adherent culture typically requires more time to scale-up than suspension culture and as a result, manufacturers look for tools to reduce scale-up time.
The iCELLis system has addressed the scale-up challenge by creating a scale-up strategy, which resembles the tactics used for scaling up chromatographic separations. The smallest units in the family—the iCELLis nano system—range from 0.53 to 4m² in surface area [Image 4], while the large-scale bioreactors measure 66 to 500m². Importantly, the media linear velocity and fixed-bed height are maintained throughout the different-sized systems, with just the bed diameter increasing, from 5cm in the smallest unit, to 71cm in the largest. Cell density can be monitored via a biomass probe at the large or Nano scale.
A further advantage is the reduced amount of material required for the initial seeding. This can be significant—anywhere between a two- to a 10-times reduction from traditional 2D supports. This lower volume is possible because the design of the iCELLis system allows the number of cells/cm² at inoculation to be reduced while the cell count per ml is maintained. High cell concentrations can be created in fixed-bed bioreactors like this because of their high oxygenation capacity; typical figures for iCELLis range from 30 million to 200 million cells per ml of fixed bed.
Reduction in Labor Requirements and Capital Investment

Another challenge in adherent cell-based manufacturing are the significant labor requirements. Labor requirements in 2D systems can be quite high, with several operators needed to run a production-scale operation where scaling up means adding many more bottles. In addition the larger footprint becomes an issue because a significant capital investment is required to install the multiple incubators and biosafety cabinets needed to house very large numbers of roller bottles. In addition adequate space needs to be obtained to accommodate a significantly larger manufacturing footprint.
In contrast, with the iCELLis unit, only one or two operators are required for operation. Plus its smaller footprint has a big impact, too—one iCELLis unit, including its controller and feed-in and feed-out tank, will fit into a 6m² space. This allows for manufacturing expansion without the need to increase the existing manufacturing footprint or incur additional capital expenditures.
Time Savings

Although the footprint required for a stirred tank reactor containing microcarriers is much lower, the requirement for preparing and sterilizing those microcarriers adds to the time it takes to prepare the manufacturing process. Perfusion is also much more time-consuming, because it involves media exchange using gravity to settle the microcarriers within the bioreactor, or an external perfusion device that also requires sterilization. The extremely long time it typically takes to develop the process is, in large part, a result of the difficulty in identifying the optimum conditions in terms of shear stress.
By circulating media using a magnetic drive impeller, low shear stress and high cell viability is maintained with the iCELLis. The fact that the iCELLis is a disposable system significantly cuts cleaning and sterilizing time investments.
Summary
A disposable fixed-bed device such as iCELLis therefore offers the perfect balance between efficiency, development time and reduction in the overall cost of production, once the optimal process conditions are identified. Ultimately, disposable fixed-bed manufacturing equipment allows development timelines to be reduced, thus speeding up time-to-market for vaccines and other products made via adherent cell culture, while optimizing the cost effectiveness of the manufacturing process.
References:
- Considering 850 cm² roller bottles.