Cell Culture Monitoring – A Critical Component for Quality by Design in Cell Therapy
Cell therapies offer much promise in treating a range of medical conditions, but manufacturing complexity is a challenge when scaling up to clinical and commercial manufacturing. Concerns include developing a process that is efficient and cost effective with consistent, high quality outcomes.
Most biopharmaceutical companies have implemented Quality by Design (QbD) and Process Analytical Technology (PAT) initiatives to address similar challenges in CHO-based biologics manufacturing. A QbD approach provides a systematic look at process development, which attempts to predict unique challenges and address them in advance. A key aspect of QbD is defining Critical Quality Attributes (CQA) and Critical Process Parameters (CPP) and then monitoring these closely through the use of PAT. The FDA has stressed the importance of their Quality by Design and Process Analytical Technology initiatives in an effort to encourage more consistent manufacturing processes and product quality.
While the path for implementing this approach in Cell Therapy manufacturing is arguably more complex, it would undoubtedly improve the process. However, for QbD to work effectively you must accurately measure and be able to maintain tight control of your culture conditions through input of reliable analytics, characterization of influencing factors, minimizing user interference and early detection of deviation. A critical first step of applying this approach in Cell Therapy is to ensure that the culture can be monitored precisely, so that critical process parameters can be accurately measured and ultimately maintained during manufacturing.
Cell Culture Monitoring to enable Quality by Design in Cell Therapy
When looking at ways to monitor Cell Therapy processes, it is good to begin by looking at how biopharma monitors biologics manufacturing. To this end, Roche Custom Biotech recently gave a Bioprocess International webcast on how to utilize their line of Cedex Bio Analyzers, frequently used in biologics QbD and PAT initiatives, in a Cell Therapy environment. In the webcast, Brett Butler, Field Application Consultant for Roche Custom Biotech, described the use of “Cedex Bio Analyzers for Optimal Control of Cell Culture Conditions” and how this specifically applies to Cell Therapy applications.
Mr. Butler began his talk by describing the importance of defining Cell Therapy culture conditions to achieve cell multiplication and maintenance of the cell-typical characteristics. Then those conditions must be maintained throughout the manufacturing process to ensure the maximum number of viable, functional cells for treatment. The Cedex Bio and Bio HT Analyzers can precisely monitor culture conditions and allow management of the culture conditions in real time to maintain an optimal defined range.
Both the Cedex Bio and the Bio HT Analyzers have a proven track record in biopharmaceutical manufacturing operations. They both provide a large number of assays for substrates and metabolites, with additional assays added regularly to respond to industry needs. The assays most applicable to Cell Therapy would include glucose, glutamine, glutamate, potassium, sodium, ammonia, lactate, and LDH.
In addition, continuous loading of samples, small sample volume requirements, and an optional pre and post dilution feature are available with both instruments. The primary difference is that the Cedex Bio HT is designed for high throughput and therefore an excellent fit for manufacturing operations.
Mr. Butler then described the way in which the Cedex Bio Analyzers can improve QbD in Cell Therapy by improving understanding of the process. He listed some key requirements in a QbD approach, these include:
- Tight control of the process
- Reliable analytics
- Characterization of influencing factors
- Early detectability of deviations
Maintaining Tight Control of a Process
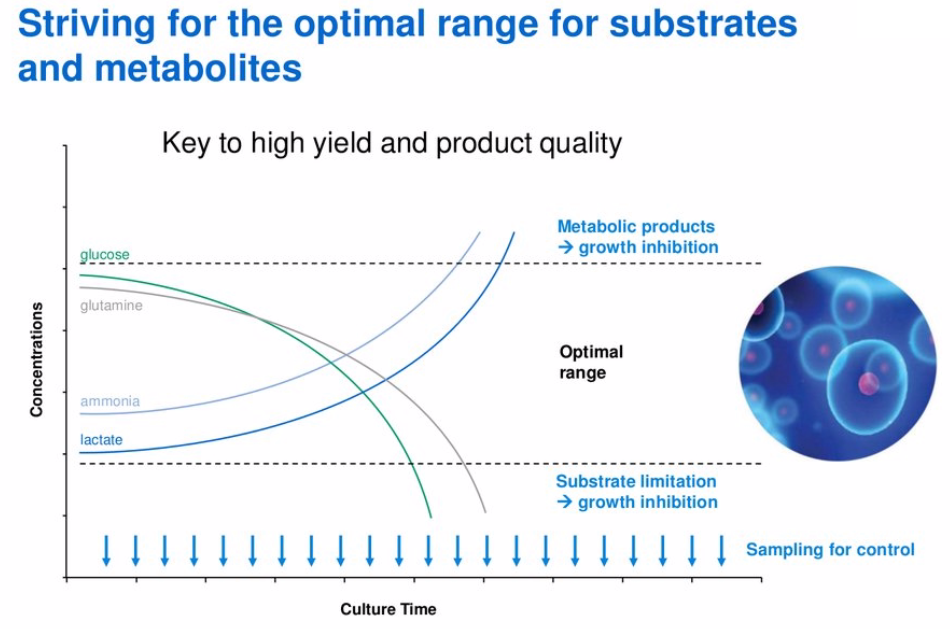
To demonstrate how the Cedex Analyzers can help to maintain tight control of a process, Mr. Butler explained the efficiency of monitoring the culture substrates and metabolites in real time. He described how as cells grow, substrates like glucose will be depleted and metabolites will increase, both conditions limit growth (Figure 1). By monitoring these during production, you can manage the concentrations and maintain them in the optimal range.
Figure 1:
To further this point, he presented data from the University of Vienna, where researchers wanted glucose to be maintained within a tight range. Whenever glucose fell below 3.0g/L, they wanted to add additional glucose to the culture. To achieve optimal glucose concentration, the Roche’s Cedex Bio Analyzer tested the culture every 12 hours. If the glucose dropped below the target amount, additional glucose was added to reach the desired level. When they compared two processes monitored with either the Cedex Bio or a membrane analyzer they found that with the membrane analyzer, the measured concentration of glucose was not as precise. In this scenario, it could mean that glucose would be added when it wasn’t truly needed or it wouldn’t be added when it was needed, see Figure 2 and Figure 3.
Figure 2:
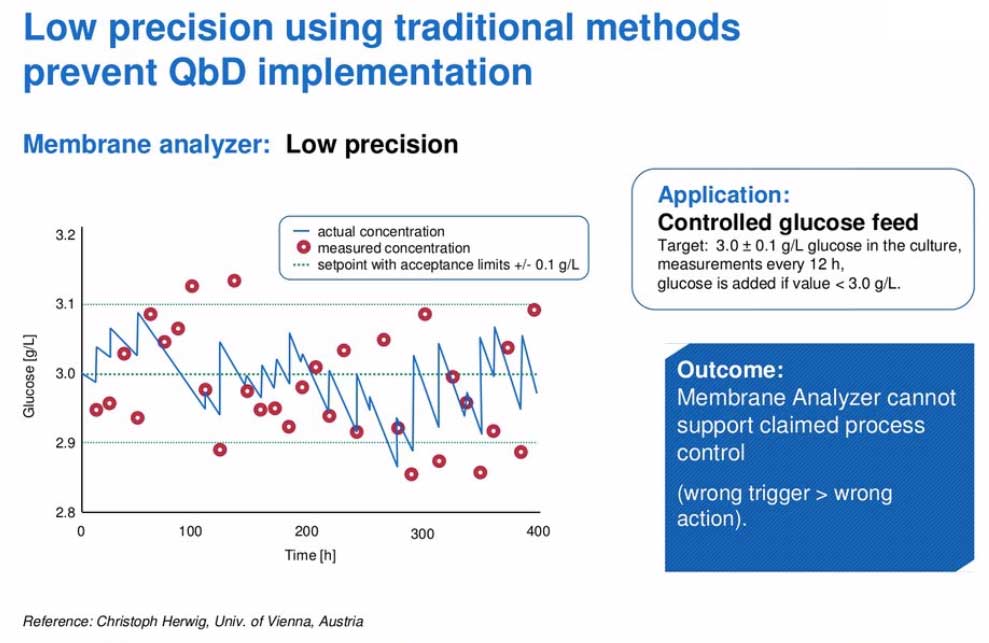
Figure 3:
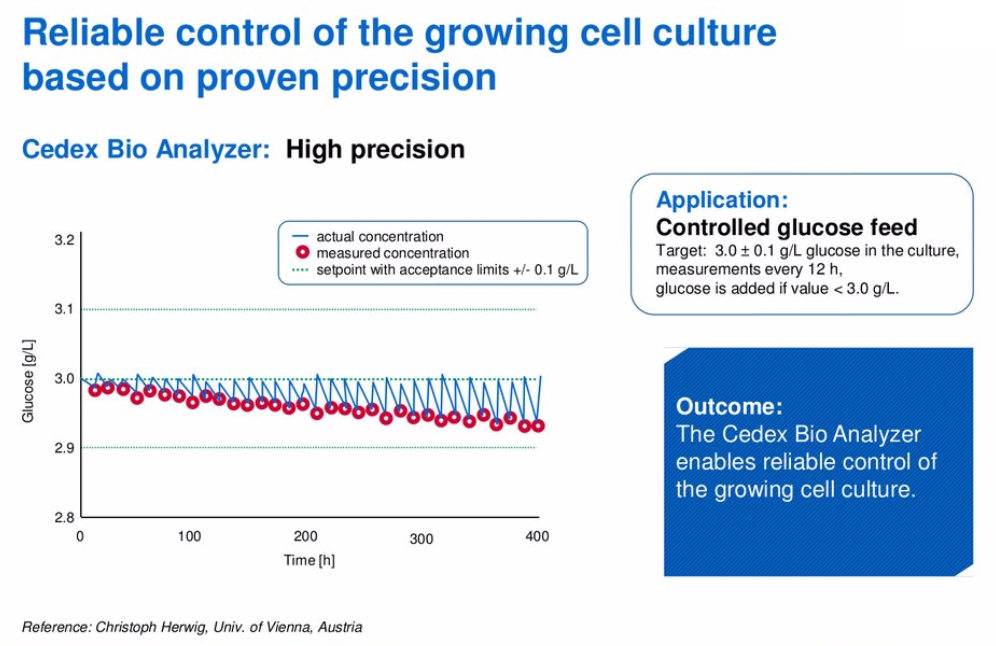
Demonstrating High Reliability
For QbD to operate successfully, there must be reliable analytics. When analytics are reliable, it enables the use of prediction models that can be used to optimize a process and/or maintain a desired process. If the analytics are unreliable then the model is incorrect and key decisions can become mistakes. An example of a proven prediction model for the glucose case study was presented (Figure 4).
Figure 4:
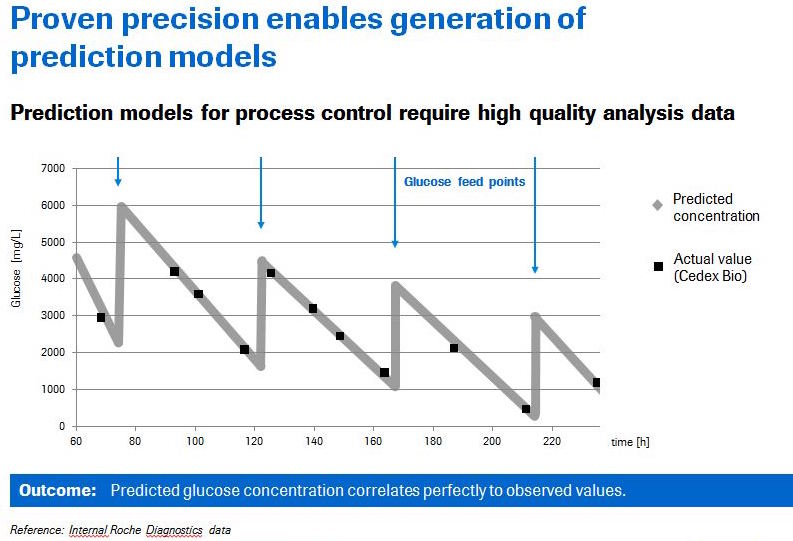
To further demonstrate the high reliability of the Cedex Bio and Bio HT, Mr. Butler presented data demonstrating equivalent results to the gold standard HPLC reference. An example of this work using a glutamine concentration assay is shown in figure 5.
Figure 5:
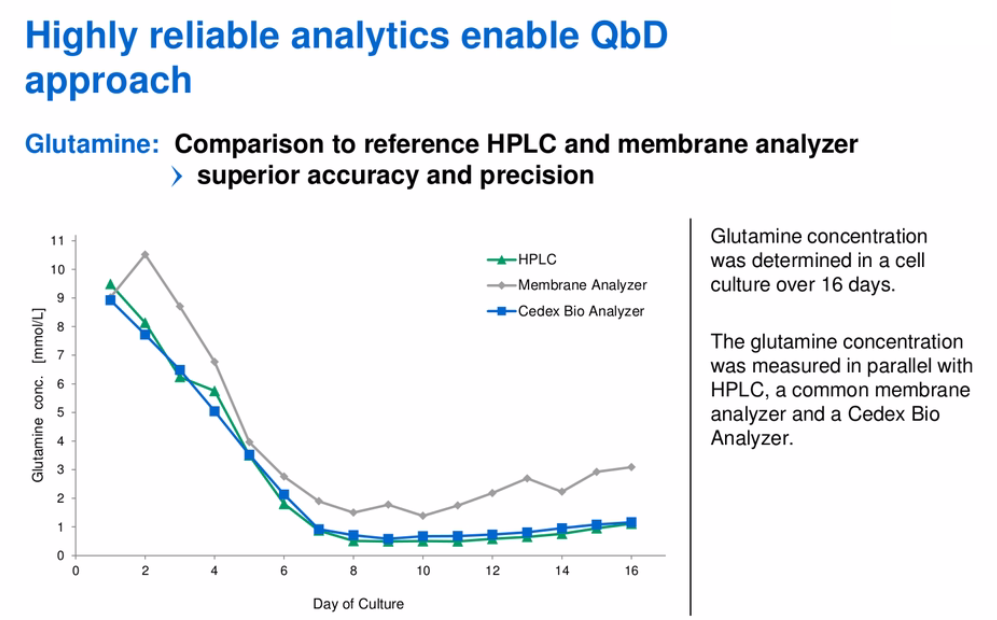
Roche Custom Biotech has also been able to demonstrate equivalence between the Cedex Bio and Bio HT, which means that scale up could be potentially seamless to high throughput as the company moves from research to manufacturing.
Media Optimization
In his next example, Mr. Butler described the use of the Cedex Bio Analyzer to develop an optimal media formulation for adult human islets. This work was conducted by Prodo Laboratories and is a summary of a comprehensive application note that was featured on Cell Culture Dish, “Application of the Roche Cedex Bio Analyzer in the culture of human pancreatic islets and adult human mesenchymal stem cells.”
The goal of the study was to extend successful culture and functional survival of human pancreatic islets to four weeks in-vitro culture. To identify the optimum culture conditions, Prodo Laboratories examined multiple parameters including glucose, lactate, ammonia and LDH. They cultured the cells for four weeks in three different media. Their Glucose (Figure 6) and LDH (Figure 7) results are shown as an example below.
Figure 6:
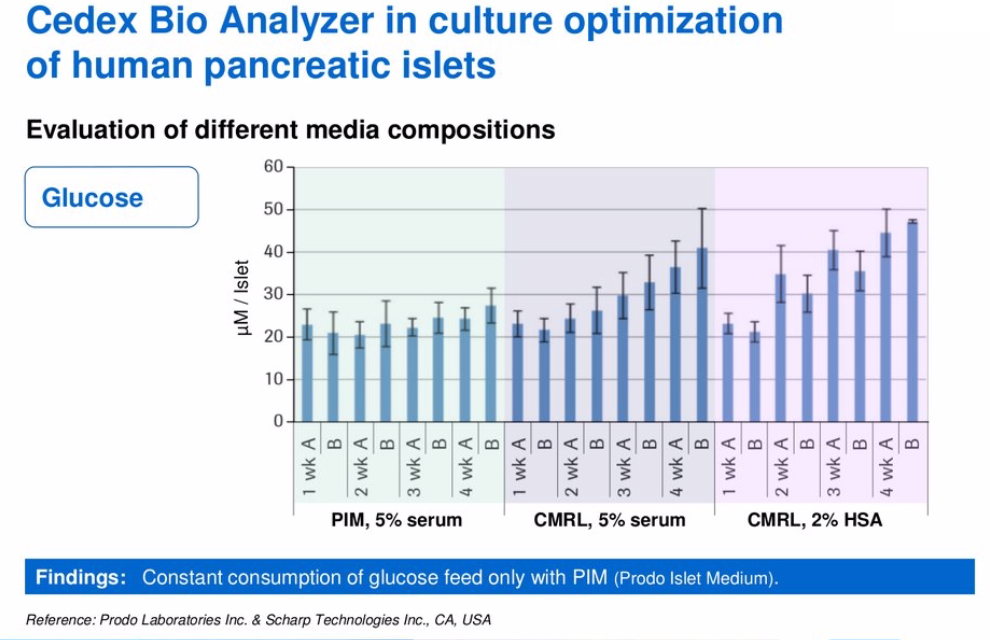
Figure 7:
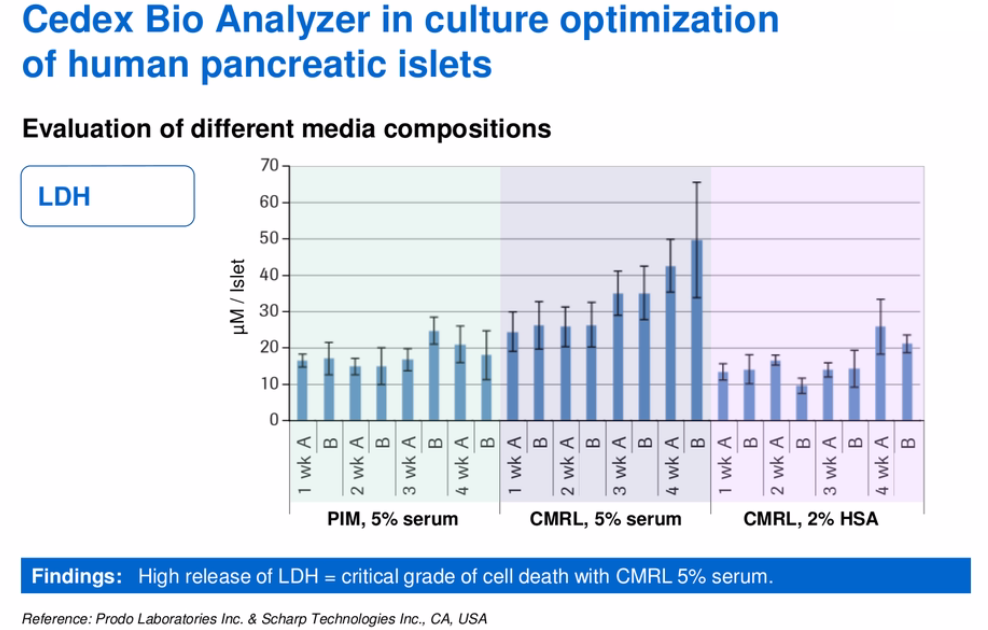
“Use of the Cedex Bio Analyzer for testing culture media from growing cells of different types provides essential information regarding the viability and function of the cultured cells under different conditions. This study suggests that the Cedex Bio Analyzer can be critical in monitoring routine primary cell cultures into long-term culture under standard production and GMP manufacturing controls. It can also be utilized in maintaining controlled culture conditions for tissue culture by measuring key metabolic substrates such as glucose, lactate, LDH, and glutamine during cell growth. Furthermore, analysis using the Cedex Bio Analyzer can provide critical information in monitoring and developing MSCs, from culture medium development and monitoring through GMP processing.” – Dr. David Scharp, Prodo Laboratories
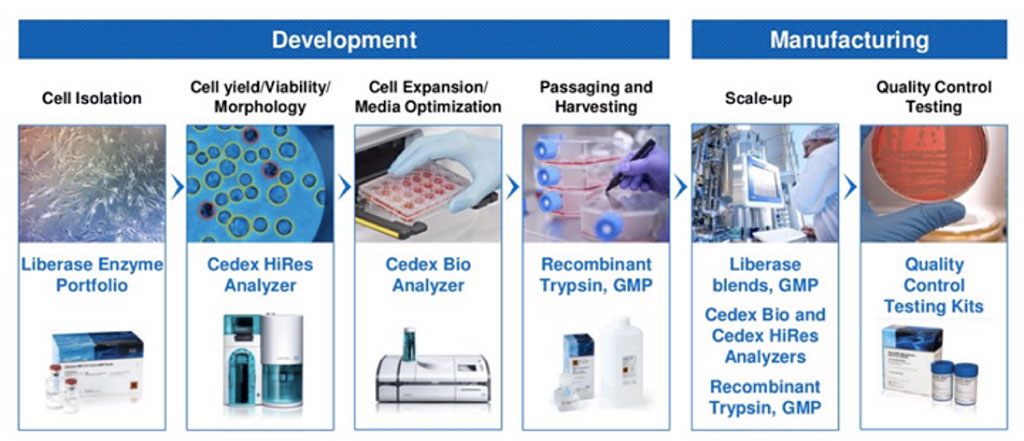
In addition to the Cedex Bio Analyzers, Roche Custom Biotech has a portfolio of products that can be utilized in Cell Therapy workflows. Learn more about Roche Custom Biotech products for Cell Therapy workflow.
Related Reading:
- Application of the Roche Cedex Bio Analyzer in the culture of human pancreatic islets and adult human mesenchymal stem cells
- Tissue Dissociation – Moving toward standardized protocols and increased consistency
Liberase and Recombinant Trypsin are for further processing only.
Cedex Analyzers and quality control testing kits are for use in quality control/manufacturing processes only.