PRIME-XV® T Cell CDM – First Commercially Available Chemically-defined, Animal-component-free Medium for T Cell Culture
Gene therapies and immunotherapies hold tremendous promise to treat diseases in a completely novel way. As increasing numbers of these therapies move from research and proof of concept to clinical trials, the manufacturing requirements will become more complex. Part of this includes a desire to move away from media that contain animal-derived and undefined components to animal component-free and chemically-defined components. In addition to the possible safety issues, animal components can be variable from lot to lot. Animal components with their naturally occurring cytokines and growth factors can also result in undesired and unexpected effects on cells.
To address these needs, Irvine Scientific recently launched PRIME-XV® T Cell CDM, the first commercially available chemically-defined, animal component-free medium for human T cell culture. The new medium has been developed to maximize consistent growth of T cells while maintaining their functionality and potency.
By removing the animal-components, the naturally occurring cytokines and growth factors that have been shown to impact growth, phenotype and the potential of T cells to polarize into therapeutic subtypes removed. This provides more consistency between lots. Chemically-defined media also reduce the risk of introducing foreign agents or impurities from undefined components, thereby facilitating scale-up to commercial production and streamlining the regulatory submission process.
Irvine Scientific lists the following benefits for PRIME-XV T Cell CDM:
- Supports vigorous T cell growth in plates, cell culture bags, and in the G-Rex® maintaining functionality
- Provides lot-to-lot consistency
- Removes the effects of undefined components on T cell phenotypes
- Supports polarization to targeted T cell types such as Th1 and T regulatory cells
Performance
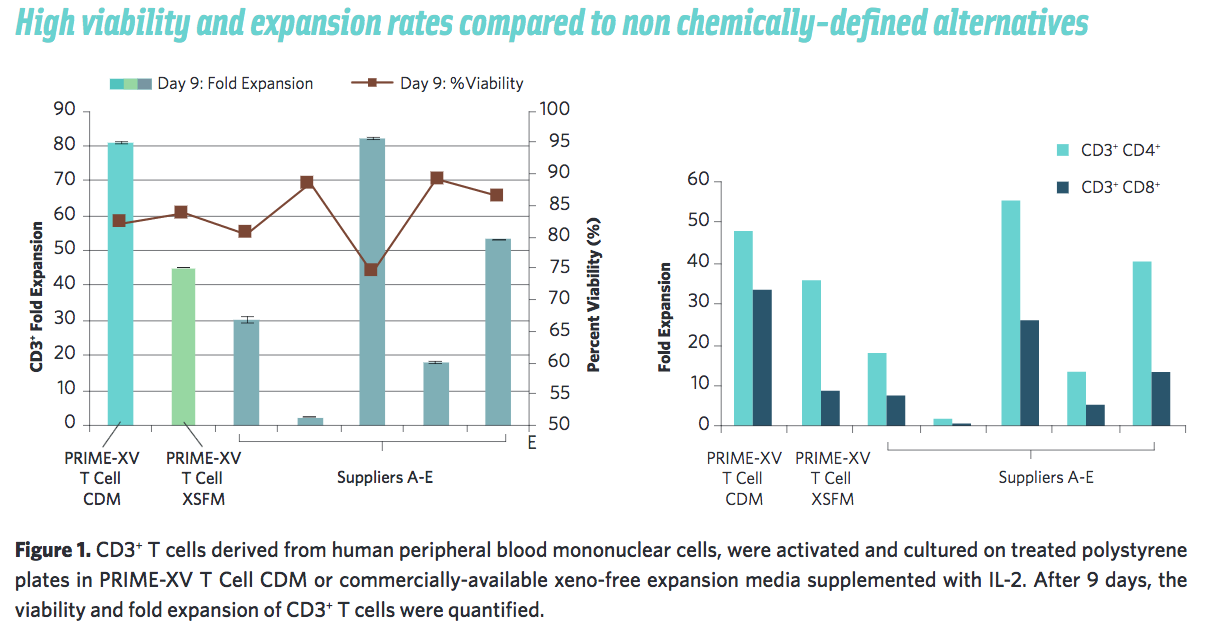
In addition to the improved safety and consistency, Irvine Scientific has presented data to show that PRIME-XV T Cell CDM performs consistently better than commercially-available alternatives and serum-based media (Figure 1).
Scalable
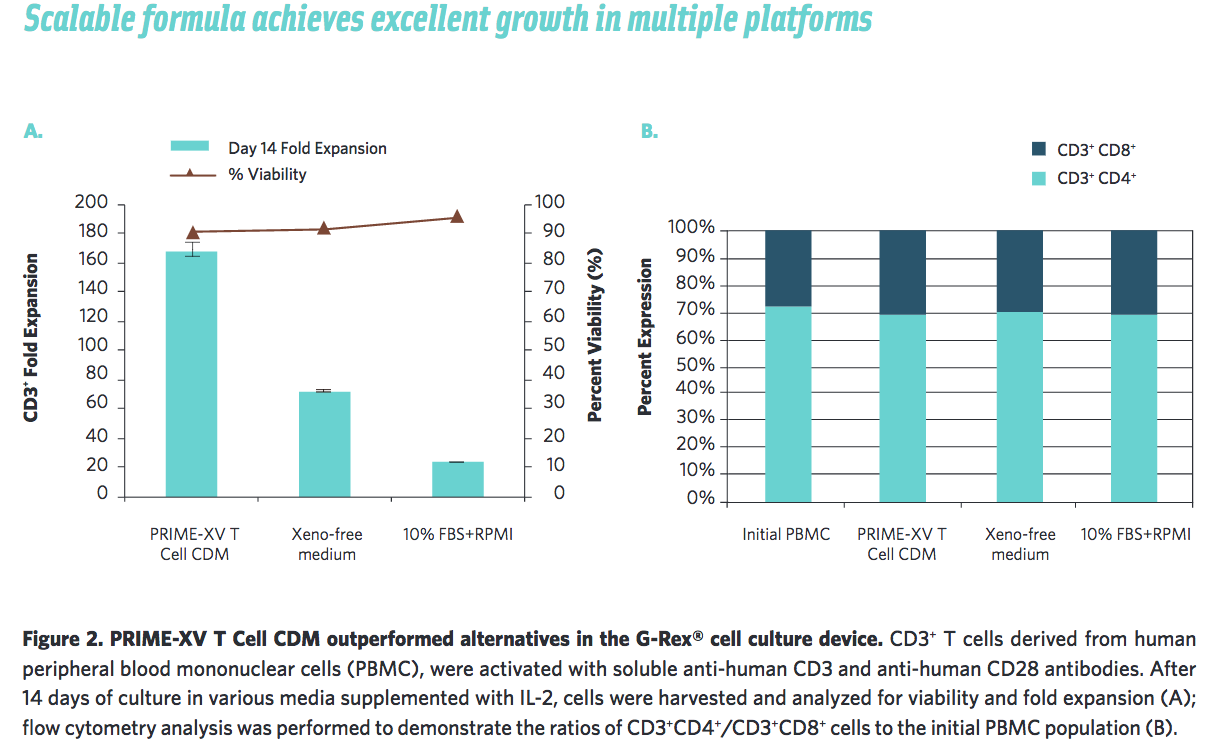
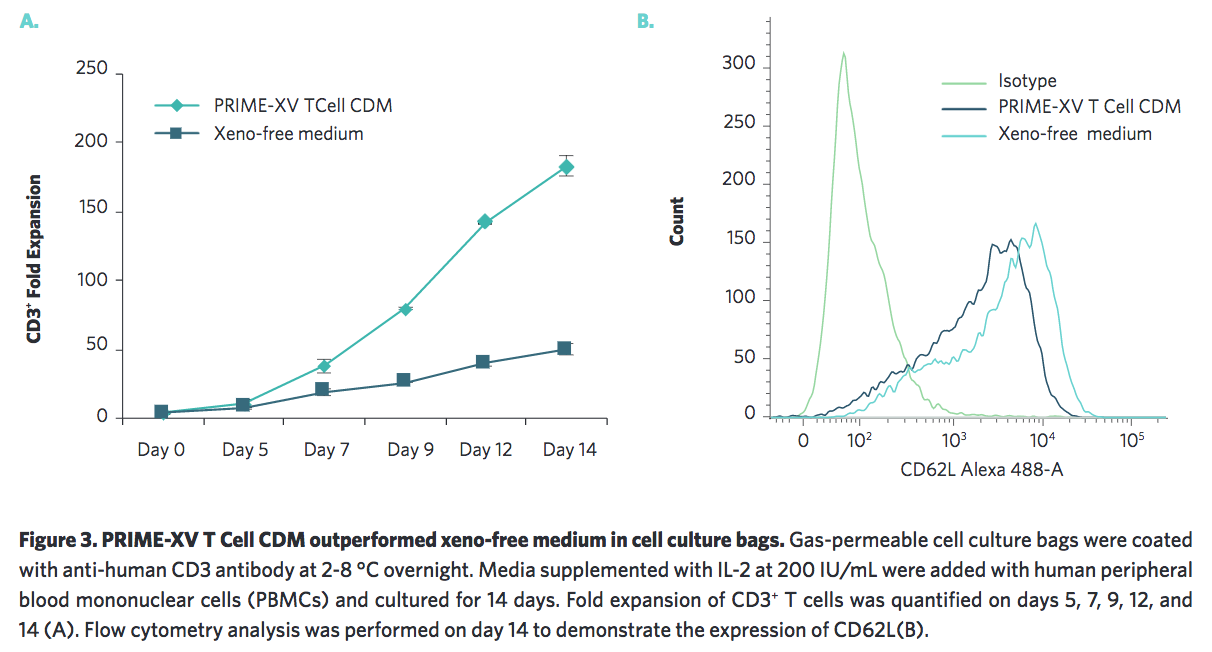
Irvine Scientific also wanted to ensure that their medium was scalable by testing in other cell culture platforms. They found that it performs well in the G-Rex system and in cell culture bags (Figure 2, 3).
Supports Polarization to Targeted T cell Types
The new media was formulated to support polarization to targeted T cell types such as Th1 and T regulatory cells (Figure 4).


Regulatory Benefits
Irvine Scientific designed the media with regulatory standards in mind to facilitate the transition from research to clinical manufacturing:
- Chemically-defined, animal component-free formula minimizes risks from adventitious agents
- Manufactured in compliance with cGMP regulations
- Traceability documentation provided including Certificates of Analysis, Certificates of Origin, and a Drug Master File (DMF) filed with the US FDA
- Extensive QA testing including functionality, sterility, and endotoxin
- Custom sizes and packaging available on request
For more product information, please visit PRIME-XV T Cell CDM
For more information or to obtain samples, please contact Irvine Scientific: Tel: +1 949 261 7800; Email: getinfo@irvinesci.com
Related Topics
PRIME-XV T Cell CDM is the ideal complement to BalanCD® HEK293 system. Introduced earlier this year, BalanCD HEK293 is a chemically-defined, animal component-free medium for the production of the viral vectors used to genetically modify T cells for Gene Therapy and immunotherapy.
A Complete System for Viral Vector and Recombinant Protein Production in HEK293 Cells