
Acoustic Cell Processing Helping to Industrialize Cell and Gene Therapy
In this article, acoustic cell processing, a unique platform technology that allows for the manipulation of cells with nearly shear-free and low power ultrasonic standing waves, is discussed with its application to cell and Gene Therapy manufacturing illustrated through case studies.
Discussion
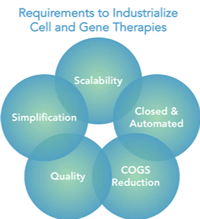
Recent success in cell and gene therapies offers hope for many patients suffering from difficult-to-treat diseases. But without new tools in the form of innovative processing equipment, manufacturers will not be able to move forward quickly to help patients. With nearly 90% of all potential therapies still in clinical phase, there is certainly the need and opportunity to industrialize the manufacturing process. Cost of goods (COGS), quality and scalability are paramount in industrializing these therapies, simplification of the process and incorporating closed & integrated platforms is also required.
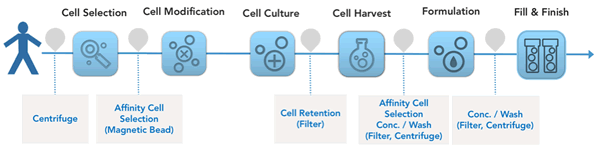
Until now a majority of the tools employed have been adapted from bioprocess, filters and centrifugation are two examples. Other technologies have been used in cell and Gene Therapy for decades with little innovation, magnetic beads for affinity cell selection is one example. Figure 1. Represents a simplified process flow for CAR-T manufacturing showing key unit operations and common technologies employed.
The end result of using these legacy technologies is a process that is not optimized, has high cost of operation due to material costs and labor and in some cases quality risk due to performance or impact on the final drug product due to the addition of reagents that are not 100% removed.
Some disadvantages of legacy technologies:
- Increased raw material costs
- Increased labor costs
- Quality impact on cells due to high shear
- Quality impact and drug product due to remaining material, i.e. magnetic beads
Is Closed and Automated the solution?
Yes and no. It seems from recent surveys and from what vendors are promoting that a closed and automated process is the solution. But that is only part of the story, it will only get you so far if the fundamental technology is inherently flawed or not optimal for the application. We learned from bioprocess decades ago that stainless steel bioreactors could be closed and highly automated with CIP/SIP and process control to prevent contamination and avoid operator error. The end result was highly complex and expensive process skids that drove up COGS. The introduction of the single-use bioreactor completely changed the model, technology solved the problem. Perhaps we should first look at the fundamental technology for processing cells in CAR-T manufacturing.
Introduction
FloDesign Sonics recognizes that to move cell and Gene Therapy forward, manufacturers must develop robust, closed, and automated commercial processes. Innovators need equipment that is flexible and adaptable to broad and evolving process needs. Solutions must also be modular so unit operations can be connected, combined, and scaled. So we developed a platform based on a proven technology which has yet to be applied to this space.
Acoustic Cell Processing is opening the door to a new vision where the specialized and expensive Cell Therapy processes of today will become routine and widespread tomorrow.
 Acoustic Cell Processing is a unique platform technology that allows for the manipulation of cells with nearly shear-free and low power ultrasonic standing waves. Ultrasonic standing waves have a variety of applications in cell and Gene Therapy. The format of the technology is flexible and modular, offering different closed acoustic elements customized to the cell number, suspension volume and performance requirements of a particular process, such as concentration, washing, affinity cell selection, and label free cell selection.
Acoustic Cell Processing is a unique platform technology that allows for the manipulation of cells with nearly shear-free and low power ultrasonic standing waves. Ultrasonic standing waves have a variety of applications in cell and Gene Therapy. The format of the technology is flexible and modular, offering different closed acoustic elements customized to the cell number, suspension volume and performance requirements of a particular process, such as concentration, washing, affinity cell selection, and label free cell selection.
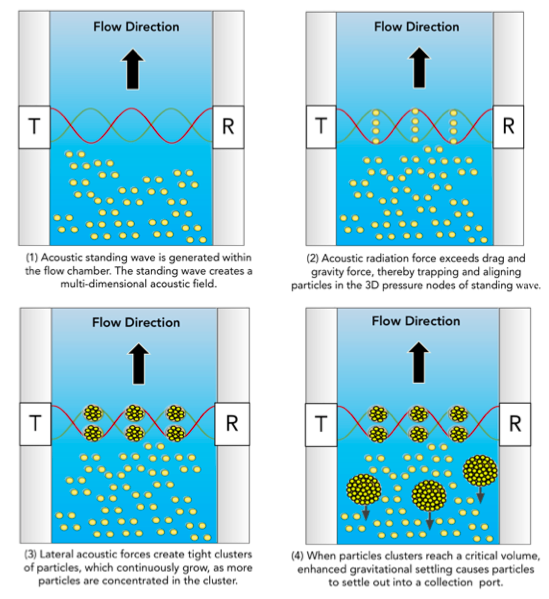
The foundation of FloDesign Sonics’ platform is multidimensional acoustic standing wave technology. A transducer generates an acoustic wave that travels to the reflector and back, generating a standing wave with locations of pressure nodes, i.e., minimum acoustic pressure amplitude, and anti-nodes, i.e., maxima of the acoustic pressure amplitude (Figure 2). When the acoustic radiation force exerted on suspended cells exceeds the combined effect of fluid drag force and gravitational/buoyancy force of the cell, the cell becomes trapped in the standing wave. The multi-dimensional nature of the standing wave continuously generates cells clusters, which will settle out when the clusters reach a critical size. This creates a simple, non-invasive way to separate materials in suspension in a closed system.
Areas we are currently advancing
- Concentrate-wash, volume reduction
- Affinity selection
- Perfusion cell retention
- Density gradient fractionation
Case Studies and Applications
Case Study: Acoustic Concentration-Wash (ACW) Concentrating Primary Cultures of T-cells
Summary
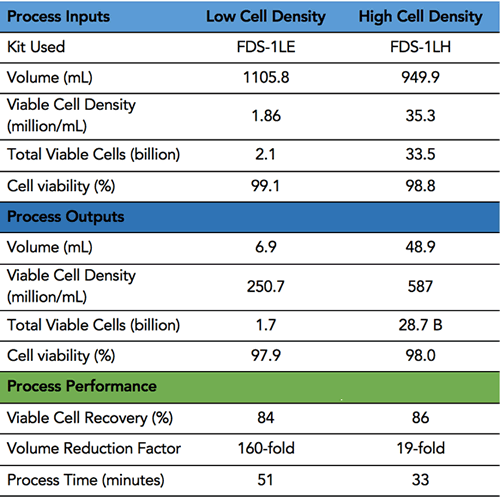
FloDesign Sonics’ concentrate/wash platform was used to concentrate cultures of activated primary T-cells. Two starting densities of the cultures were tested; the first a low cell density on the order of 1M/ml and the second a high cell density 30-fold higher in concentration. The concentration test of the low cell density culture is typical for a pre-electroporation concentration process. The high cell density experiment is typical for a harvest concentration process.
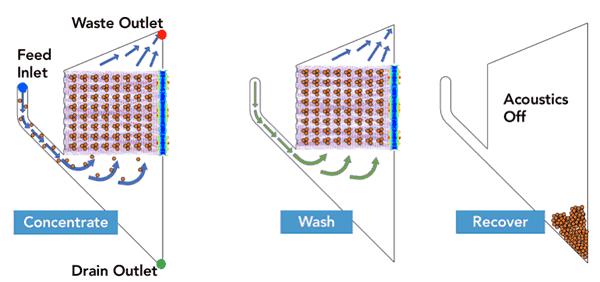
FloDesign Sonics’ universal electronics platform was used to excite the transducer and control the process. Two different single-use acoustofluidic chambers were used; one sized for the low cell density culture and a second for the high cell density culture (FDS-1LE and FDS-1LH). The acoustic chambers were designed to meet the desired throughput and output requirements of final cell density of the concentrated cells and total volume of the concentrated cell solution. In the first step the acoustic field is used to hold cell within the ACW element while waste material (fluid) is pulled off. In the second step the cells are still held in the acoustic field while washing solution is passed through the ACW element. Finally, the acoustic field is turned off and the cell settle out (Figure 3). Results are found in Table 1.
ACW Results
Study Shows a 160-fold reduction factor and an 84 percent yield of viable cells with no significant loss in viability
Case Study: Acoustic Affinity Cell Selection (AACS)
Summary
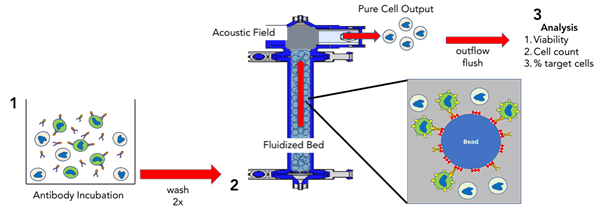
FloDesign Sonics’ AACS platform has been developed to efficiently separate a high volume of T-Cells using a fast, non-magnetic approach for CAR-T manufacturing (Figure 4). After antibody incubation cells are introduced to a fluidized bed containing proprietary, non-magnetic beads. An acoustic field at the top of the fluidized bed retains the larger cell / antibody / bead assembly. After negative selection, target cells pass through the acoustic field and exit the fluidized bed.
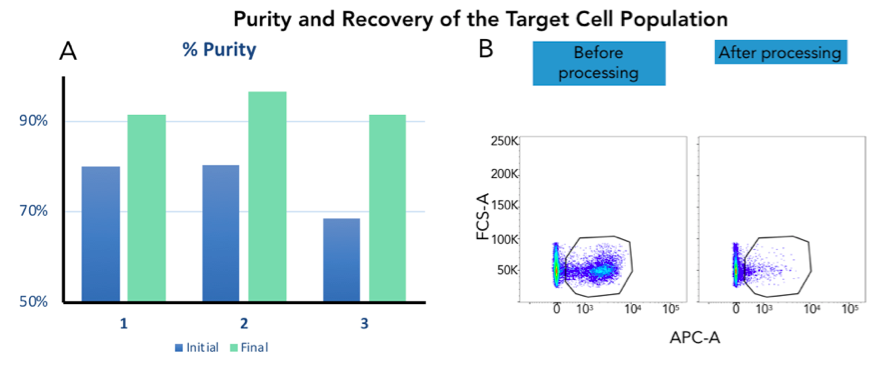
AACS Results
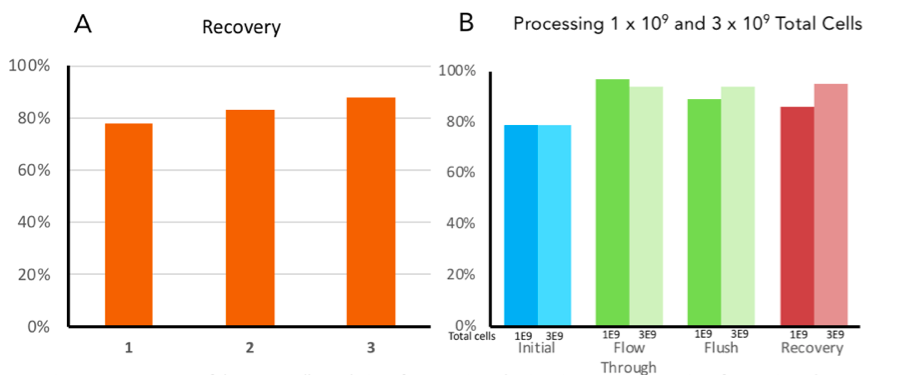
Future Applications
- Acoustic Activation/Transduction/Transfection
- Cell Enrichment/Fractionation
- Cell Retention in Cell Culture
The previous case studies highlight two mechanisms of manipulating cells using acoustic wave. These same mechanisms could be applied to viral vector applications where there is the need for intimate interaction between virus, cells, and beads. By changing the angle of the acoustic wave across the element, cells populations can be divided or enriched to target desired cell ratios. Finally the same acoustic field principle applied in the AACS application could be used in a purposed built assembly to hold back cells in a bioreactor as typically done in perfusion applications.
Conclusion
Acoustic Cell Processing enables cell and Gene Therapy developers to make the critical transition from manual high touch open operations to closed, automated and single use commercial processes. The “acoustic engine” at the heart of FloDesign Sonics’ modular platform has been proven in several critical areas in the CAR-T manufacturing process. This same technology is now being applied to many other applications in cell and Gene Therapy. The acoustic platform has inherent technical advantages over traditional processes such as filtration, centrifugation or magnetic beads in affinity separation applications. Finally, the modular platform combined with acoustics facilitates a closed, automated platform which can gently process cells during continuous operation. These characteristics combined will help industrialize the manufacturing of cell and gene therapies by providing:
- A robust, scalable platform
- Closed, automated operation
- COGS reduction
- Improved quality
- Simplified operation
To learn more about acoustic cell processing, please visit FloDesign Sonics.
About the Author:
 Rui Tostoes, PhD, Vice President Bioprocessing, FloDesign Sonics
Rui Tostoes, PhD, Vice President Bioprocessing, FloDesign Sonics
Dr. Tostoes has extensive experience in early stage device and process development for Cell Therapy manufacturing. In previous work, he has cultured human primary and stem cells in bioreactors, developed a new filtration device for cell washing, and worked on a cell manufacturing process for Phase I trials. While at the Center for Commercialization of Regenerative Medicine in Toronto, Dr. Tostoes was the technical lead in bioreactor process development, and worked with both human pluripotent stem cell culture and new inline sensor technologies for Cell Therapy culture. Currently, he leads the Cell Therapy process development at FloDesign Sonics in Co-Development agreements with Cell Therapy customers. He has performed studies in other bioprocess unit operations, including cryopreservation and using high throughput and scale down methodologies. Dr. Tostoes received his PhD in bioengineering from Nova University, Massachusetts Institute of Technology and did his post doctoral work in Biochemical Engineering at University College, London.