Stromal Cell Isolation from Adipose Tissue
Comparative Analysis using Roche Liberase MNP-S and Worthington Collagenase I
A Guest Blog by Authors: Stephanie Merfeld-Clauss, Dmitry Traktuev, Keith March
Department of Medicine; Indiana Center for Vascular Biology and Medicine, Indiana University School of Medicine; VA Center for Regenerative Medicine, R.L. Roudebush VA Medical Center, Indianapolis, IN 46202
Introduction
The idea of Cell Therapy using the systemic or local injection of stem/progenitor cells in the area of injury to treat multiple chronic disorders received close attention in the last decade.(1) Bone marrow-derived mesenchymal stem cells (BM-MSC) and blood-derived endothelial progenitor cells were among the first cell types that were evaluated in regard to their therapeutic potentials.(2,3,4)
Adipose stem/stromal cells (ASC) represent another type of mesenchymal cell and, in contrast to BM-MSC, are substantially more abundant in adults.(5) BM-MSC and ASC are among the clinically feasible and promising candidates for autologous Cell Therapy applications due to their pluripotency, immunomodulative properties, and secretory activity.(6,7) Therefore, they are the subject of study in several Cell Therapy clinical trials worldwide. The most efficient way to generate a stromal vascular fraction (SVF) and then extract ASC from adipose tissue is through dissociation of a lipoaspirate by enzymatic digestion. Currently there are many different products on the market that are suitable for adipose tissue dissociation, many of them containing a blend of collagenase I (isolated from C. histolyticum cultures) and a neutral protease, such as dispase.
Multiple preclinical studies have demonstrated that dissociation of adipose tissue with collagenase alone or in combination with neutral protease results in efficient extraction of viable and proliferative ASC, and that, post expansion, these cells are capable of multilineage differentiation into adipocytes, chondrocytes, osteocytes, and smooth muscle cells.(8)
While most blends are acceptable for preclinical and basic science studies, the more strict requirements for clinical trials significantly narrow the options. Factors such as lot-to-lot enzyme activity inconsistency, insufficient purity from endotoxins, other protease activity, and animal-derived components raise safety and applicability concerns for clinical use. In order for the reagent to be accepted as an in-process reagent for the preparation of cells for transplantation, it must be GMP grade with standardized final blend purity and lot-to-lot consistency.
Our laboratory conducted a study to compare side-by-side human adipose tissue dissociation to single-cell suspension using crude, non-completely characterized Collagenase I (Worthington Biochemical Corporation) and Liberase MNP-S, a Sterile-A GMP-grade blend of collagenase I and II with a medium content thermolysin component (Roche Diagnostics). Highlights from the study are below, with full information available here.
Materials and Methods
Isolation and Expansion of Cells
Tissue samples were collected in sterile containers during scheduled abdominal liposuction procedures. In the first part of the study, 10 ml of lipoaspirate was aliquoted into each of four 50 ml tubes. An equal volume of Liberase MNP-S at 0.9 Wünsch units/ml was added to each tube. Tubes were incubated in an Enviro-Genie incubator with a rocking speed of approximately 40 cycles per minute at 37°C for 10, 20, 30, and 40 minutes. In the second part of the study, 25 g of lipoaspirate, which was approximately 25 ml of tissue suspension, was aliquoted into each of six 50 ml tubes. An equal volume of Liberase MNP-S at 0.9 Wünsch units/ml or of Collagenase I at 2 mg/ml was added to the tubes. In total, three lots of each enzyme were tested. Tubes were incubated in an Enviro-Genie incubator with a rocking speed of approximately 40 cycles per minute at 37°C for 30 minutes.
Following incubation, dissociation tubes were centrifuged at 300 x g for 8 minutes to separate the stromal cell fraction (pellet) from adipocytes. The cells were resuspended in EBM-2/5% FBS media, filtered through 100 μm-pore membranes, and again centrifuged at 300 x g for 8 minutes. These pellets were resuspended in red-cell lysis buffer, incubated at 37°C for 10 minutes, diluted in double volume of EBM-2/5% FBS media, filtered through 40 μm-pore membranes, and centrifuged at 300 x g for 8 minutes. Pellets were resuspended in EGM-2MV media, counted manually using a hemocytometer, and plated on tissue culture plastic. For culturing: 100,000 of cells were resuspended in 2 ml of EGM-2MV media, plated into one well of a 6-well plate, and placed into an incubator with 5% CO2 and 37°C. Medium on the cells was changed after 24 hours and then every three days. ASC were passaged when 60-80% confluent. Cell monolayers were treated with 0.05% Trypsin solution for 5 minutes at 37°C to the lift the cells. ASC were used at passage 1 for follow-up tests.
Analytical Methods
The following methods were employed to compare characteristics of the resulting cell populations isolated with Collagenase I and Liberase MNP-S.
- Analysis of stromal vascular fraction (SVF) composition by flow cytometry
- Analysis of the adipogenic potential of the adipose stem/stromal cells (ASC)
- Analysis of the vasculogenic potential of the ASC
Quantitative data are expressed as mean ± SEM. Comparisons between groups were performed with an unpaired t-test. Each experimental condition represents at least n = 4. Statistical analysis was performed using Prism 4 (GraphPad). Our prior studies have shown that SVF and ASC yields may be substantially different between samples. This is probably dependent upon several factors, including donor age, BMI, region of tissue harvest, and method of its extraction. The significant variability in cell yield between samples makes it difficult to perform statistical analyses of the collective data and to graphically present it. To overcome this problem, in the graphs representing collective data, the cell counts for Collagenase I-treated samples are presented as 100%.
Study Results
Side-by-side Comparison of SVF Yield, Composition, and Cell Activity
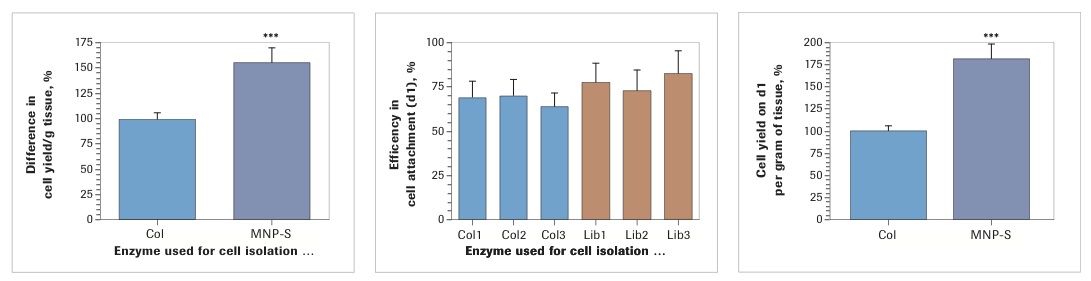
Analysis of cell yield after enzymatic dissociation of the lipoaspirate with three lots of Worthington Collagenase I and three lots of Roche Liberase MNP-S revealed that in both cases there was a low, seemingly insignificant, level of lot-to-lot variability. At the same time, when evaluated collectively, isolation with Liberase MNP-S gave consistently higher cell yields (Collagenase I: 100±5.81%; Liberase MNP-S: 156.8±13.6%, p<0.001). Analysis of efficiency of cell attachment, performed on day 1 post isolation/plating, demonstrated insignificant differences between the enzymes (Collagenase I: 67.3±4.9%; Liberase MNP-S: 77.4±6.3%). However, because the yield of SVF was higher in every Liberase MNP-S-treated sample, the total number of attached cells per gram of lipoaspirate was substantially higher for Liberase MNP-S than for Collagenase I (Collagenase I: 100±6.0%; Liberase MNP-S: 181.6±17.7%, p<0.001).
Characterization of SVF Composition Based on Surface Market Expression
It is well known that SVF is a highly heterogeneous cell population, composed of adipose stromal/stem cells, endothelial cells, hematopoietic cell types, pericytes, and other cell types. The most interesting cells in SVF, in regard to clinical use, are ASC. Multiple surface markers are used to characterize cells within these populations. We defined ASC as CD34+/CD45-/CD31- cells or as CD140b+/CD45-/CD31- cells. Flow cytometry-based analysis revealed that the percentage of CD34+/CD45-/CD31- cells in SVF obtained with Collagenase I digestion was 33.0±2.4% and with Liberase MNP-S was 39.0±2.5%. The percentages of CD140b+/CD45-/CD31- were 23.9±3.1% and 26.7±2.8% for Collagenase I and Liberase MNP-S, respectively.
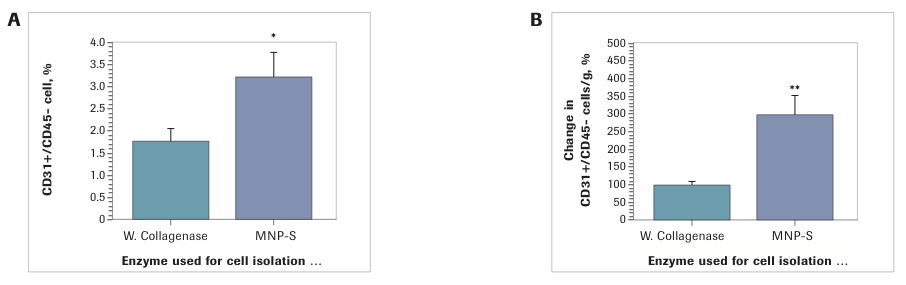
The percentage differences between enzymes are insignificant. However, because the total cell yield with Liberase MNP-S was higher, the number of ASC per gram of lipoaspirate was significantly higher. Specifically, independent from the way the ASC were defined in SVF (CD34+/CD45-/CD31- or CD140b+/CD45-/CD31- cells) there was a 90% increase in ASC yield with Liberase MNP-S (CD34+/CD45-/CD31-: Collagenase I: 100±7.1%; Liberase MNP-S: 189.3±19.1%, p<0.001); CD140b+/CD45-/CD31- cells: Collagenase I: 100±11.4%; Liberase MNP-S: 191.1±25.4%, p<0.01). Further SVF analysis revealed that samples isolated with Liberase MNP-S had twice the number of endothelial cells (3.2±0.6%), defined as CD31+/CD45- cells, compared to the SVF isolated with Collagenase I (1.8±0.3%)(p<0.05), which resulted in a three-fold increase in yield of EC per gram of SVF by Liberase MNP-S isolation (Collagenase I: 100 ±8.0%; Liberase MNP-S: 297.9±54.8%).
Discussion and Conclusions
We saw a high lot-to-lot consistency for both tested enzymes to successfully isolate SVF. However, at the same time, we consistently saw a significantly higher cell yield for all tested lots of Liberase MNP-S. The efficiency of cell attachment was similar for both enzymes, but the higher cell yield with Liberase MNP-S resulted in much higher (up to 80%) yields of ASC – primary cells of interest – per gram of lipoaspirate. Evaluation of SVF composition revealed that percentages of ASC in the SVF, defined as CD34+ or CD140b+ but CD45-/CD31- cells, were the same for both enzymes, but Liberase MNP-S was more efficient in extracting endothelial cells from the tissue.
When isolating cells from tissue, one must always be concerned with whether the use of different enzymes might result in variations in the cell types, subsequent cell behavior, and in vitro and in vivo activity, such as mitogenic activity or differentiation potency. Analysis of cell activity after extraction with Collagenase I or Liberase MNP-S suggests that both methods of isolation gave rise to cells with indistinguishable physiological activities: cells have the same rate of proliferation, degree of adipogenesis, and potency to support endothelial cell reorganization into vascular-like structures, a process that relies on ASC paracrine activity and direct heterogeneous cell-cell interaction.
In conclusion, this study demonstrates that dissociation of lipoaspirate with Roche Liberase MNP-S to single-cell suspension is more efficient than dissociation with Worthington Collagenase I. Resultant cell population characteristics are virtually indistinguishable, but the efficiency of isolation with Liberase MNP-S in regard to getting higher yields of viable biologically active ASC as well as endothelial cells, suggests it is a better choice. This data, combined with the fact that Roche Liberase MNP-S is GMP-grade with high lot-to-lot consistency, makes the product well-suited for SVF isolation in clinical applications.
Click here to read the full study.
Click here to learn more about Roche enzymes for tissue dissociation.
Roche offers a comprehensive portfolio of tissue dissociation enzymes, in both research and GMP grades, to facilitate a smooth transition from research to downstream clinical applications.
Footnotes
-
1. Botham CM, Bennett WL, Cooke JP. Clinical trials of adult stem Cell Therapy for peripheral artery disease. Methodist Debakey Cardiovasc J. 2013;9:201-205.
-
2. Iafolla MA, Tay J, Allan DS. Transplantation of umbilical cord blood-derived cells for novel indications in regenerative therapy or immune modulation: A scoping review of clinical studies. Biol Blood Marrow Transplant. 2013;20(1):20-5.
-
3. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010 Aug 15;12:87-117.
-
4. Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009 Nov-Dec; 13(11-12):4385-402).
-
5. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: A review of current clinical and translational applications. Arch Plast Surg. 2013;40:666-675.
-
6. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724-2752.
-
7. Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent cd34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77-85.
-
8. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211-228.