
Enabling High Density Cell Banking using a Single-Use, Closed-System
Novel thermoplastic tubing (FP-FLEX) coupled with single use freezing bags make processing and freezing to temperatures -196° C possible for commercial working cell banks.
Introduction
Working cell banks are typically the first step in any biotherapeutic manufacturing campaign. Thus their efficiency is key in getting product manufactured in the shortest timeline possible. In recent years the industry has turned attention toward increasing the efficiency of cell banking, inoculation and seed train scale up. Key areas identified as areas for improvement include:
- High density cell banking with banking volumes up to 1 liter
- Closed systems to reduce the risk of contamination and manual operations.
Areas for Improvement
High density cell banking
Where conventional seed train starts with thawing banked cryovials, then scaling these cells up in successively larger volume vessels until the appropriate seeding density is reached for bioreactors; high density cell banking begins with larger volume frozen seed banks up to 1 liter, typically in bags. This allows for the elimination of the intermediary steps between cryovial thaw to bioreactor and permits seeding directly into the bioreactor. Advantages of this method include consistent inoculation across process runs and the ability to eliminate steps in the seed train process thereby reducing time to bioreactor.
Closed systems to reduce the risk of contamination and manual operations
Closed systems are processes where there are no open steps. With conventional seed train expansion you have a scaling up from cryovial to larger culture vessels before reaching bioreactor seeding and in this scale up many steps that involve opening containers and manual operations. These open and manual steps increase the risk of contamination and operator error or inconsistency. By utilizing single-use bags and tubing these initial steps can be eliminated and a closed system can be established for seeding directly from the cell banking bag to the bioreactor through sterile connectors.
Challenges
Current cell banking storage options are not ideal. While cyrovials are the most common method, they are not optimal for commercial manufacturing. The key issues with cyrovials are that they are small, require significant manual operation of opening/closing the screw cap, which is both time consuming and creates an open process.
In an effort to improve on this system, single-use bags have been explored as an attractive solution. They provide a disposable closed system, thus addressing concerns of contamination and reducing manual operations. They also enable storage of larger volumes and the opportunity for high density cell banking. However the challenge has been that either the bags themselves or the attached tubing can’t withstand the low cryogenic temperatures required.
A System To Enable Improvements in Working Cell Banks
To address these problems FP-FLEX™ was launched last September by Charter Medical. In a recent Bioprocess International webcast, Dominic Clarke, Global Product Manager, Charter Medical describes the “Development of a Novel Thermoplastic Tubing, FP-FLEX™ and Single-Use Freezing Bag for Working Cell Banks Enabling Closed-System Processing to Temperatures as low as -196°C.”
In the webcast, Dr. Clarke, gives a very informative presentation about the innovative FP-FLEX™ thermoplastic tubing and what it can provide in terms of improving working cell banks. First Dr. Clarke walked through a typical cell expansion process and the challenges of current processes including open and multiple process handling steps, which increase the risk of failure or contamination of the process. He then discussed current storage options and the challenges associated with each:
- Cryovials – (1mL – 5mL) small volume, open system, multiple handling steps required
- Cryogenic storage bags – (10mL – 200mL) most common for hematopoietic progenitor cells, small volume and PVC tubing is not freeze friendly, EVA tubing is not weldable. Also they have a spine port system, which is not a closed system.
- Bulk storage bags – (25mL – 16L+) most commonly used for bulk storage and tubing and ports are not typically rated for LN2, tubing is not united.
Dr. Clarke identifies that better options were needed in order to take advantage of the benefits of bag-based storage. As a result, Charter Medical developed the FP-FLEX™ cold chain tubing and Freeze-Pak™ cryogenic storage containers that are specifically designed for ultra-low temperature frozen storage and transport applications, which enable a closed process for working cell banks. Dr. Clarke also compares the traditional seed train expansion system with the system using FP-FLEX™ and Freeze-Pak™. Figure 1 demonstrates the time that can be saved by eliminating initial scale up steps (Figure 1).
Figure 1
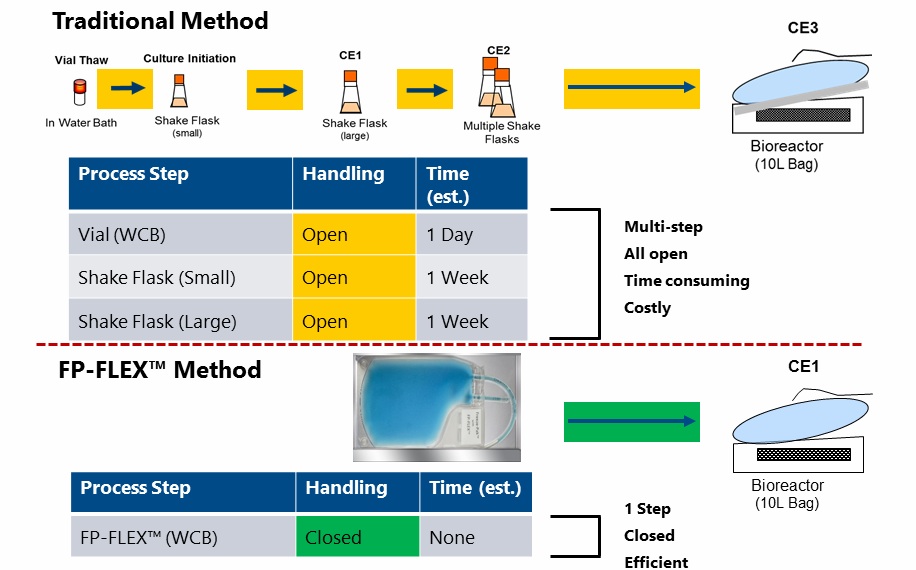
In the webinar Dr. Clarke goes on to describe the specifics of the FP-FLEX™ cold chain tubing and Freeze-Pak™ cryogenic storage bags and the validation studies conducted to ensure that they were appropriate for their intended use. I have captured some of the highlights here, but please see the full webinar for all details.
- Thermoplastic tubing available in ¼” by ⅛”
- Tubing is unitized to the Freeze-Pak™ Bag
- Validated for use to LN2
- Weldable to C-Flex® (post thaw)
- Compatible with standard welders/sealers
- The bags come in a variety of sizes – 50mL to 1,000 mL
Dr. Clarke went on to describe the validation testing that was conducted. The testing demonstrated that the products passed sterility, dye penetration and microbial challenge. In addition for weld functional testing all samples passed integrity, flow rate and weld strength requirements. Drop testing was also conducted and confirmed that the design of bags was sufficient to ensure utility after use under intended process conditions. Lastly all samples passed transportation testing. A full validation package is available upon request.
More information about validation testing is also available in the poster – Development of a Novel Thermoplastic Tubing, FP-FLEX™ and Single-Use Freezing Bag for Working Cell Banks Enabling Closed-System Processing to Temperatures as low as -196°C.
