Adipose- and Bone Marrow-Derived hMSCs: What’s the Difference?
A Guest Blog by RoosterBio
Introduction
Human Mesenchymal Stem/Stromal Cells, or hMSC, are key components of future therapeutics, engineered tissues, and medical devices and are currently in use in over 400 clinical trials (1). Bone marrow-derived MSC (hBM-MSC) have historically been the most widely used hMSC, but hMSC can be isolated from many tissues of the body including fat, umbilical cord blood, dental pulp, Wharton’s jelly, and peripheral blood. In recent years, human Adipose (or fat) tissue-derived MSC (hAD-MSC) are increasingly used in studies due to adipose tissue having a higher frequency of MSC than bone marrow and the relative ease of collection (2). You can find more information on hMSC by following these links:
- What are MSCs
- MSCs: The Workhorse of Regenerative Medicine
- Widespread MSC Misconceptions
- Using MSCs to Beat Cancer
A common MSC misconception is that MSC isolated from different tissues are equivalent. hAD-MSC and hBM-MSC, and cells from other tissues, can meet the “traditional” ISCT criteria to identify a cell as an MSC (3,4): adherence to plastic, characteristic surface marker expression profiles (positive for CD73, CD90, CD105; negative for CD34, CD45), and trilineage differentiation to fat, bone, and cartilage. However, there is widespread acceptance that hMSC achieve their biologic and therapeutic effects in vivo by secreting many bioactive molecules (referred to as the hMSC secretome) that moderate a variety of processes including angiogenesis, immunosuppression, and overall “tissue repair” (5). Despite being similar overall, hMSC isolated from adipose and bone marrow display some differences in functional capabilities (2,6). For example, hBM-MSC are more robust in bone and cartilage differentiation than hAD-MSC and hAD-MSC are more efficient at stimulating angiogenesis than hBM-MSC (2,6,7).
We have recently been applying our manufacturing protocols to adipose-derived hMSC (our newest product) and would like to share some of the similarities and differences in function between hBM-MSC and hAD-MSC that we have observed when these cells are cultured in our media systems with our protocols. Both populations of hMSC have been manufactured using our GMP-compatible and scalable manufacturing processes, with standardized procedures and with rigorous quality control. By reporting the differential functional characteristics of these hMSC populations, we assist our customers in making more informed choices on the cell type best-suited to their application(s).
Methods and Experimental Design
Materials & Reagents
Cell culture reagents, excluding RoosterBio materials, were purchased from Life Technologies, chemicals and reagents for kynurenine measurement were from Sigma, and cultureware was from Corning. Two vials (1 million cells each) of hAD-MSC, representing two donors, were purchased from ZenBio, and used only for comparison. Other cell products used were RoosterBio hMSC products: Bone Marrow-derived MSC (hBM-MSC, part # MSC-001, MSC-003) and Adipose-derived MSC (hAD-MSC, part # MSC-020, MSC-021). Cells were cultured in RoosterBio High Performance Media (part # KT-001) or DMEM + 10% FBS
Methods
All methods for the analyses shown below are documented under RoosterBio’s Quality Control systems. For more information, please contact us at info@roosterbio.com. Detailed methods for priming hMSC can be found in a previous blog post here.
Results
It is important to note that the data provided is from a limited number of donors, and that the adipose and bone marrow are not donor paired. Thus, the results presented are likely part donor variability and part tissue variability. The extent of each will only be determined over time.
hAD- and hBM-MSC display similar cell surface marker profiles
We analyzed the cell surface markers expressed on hBM-MSC and hAD-MSC by flow cytometry. Both hMSC populations were low for hematopoietic stem cell markers, CD45 and CD34 and >90% positive for MSC markers CD166, CD105, and CD90 (3,4). As the marker expression has been shown to have little correlation with cell function, we are focusing this blog on the functional markers discussed below.
hAD- and hBM-MSC are both capable of rapid cell growth in RoosterBio media
A key characteristic of RoosterBio hBM-MSC is rapid cell growth in RoosterBio High Performance Media (>10-fold expansion
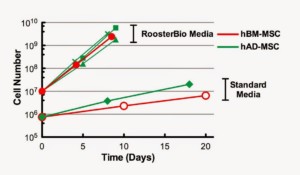
in 5-7 days). We compared growth of hBM-MSC (one cell lot shown) to hAD-MSC (RoosterBio and ZenBio) in RoosterBio High Performance Media and in a traditional hMSC growth medium (DMEM +10% FBS). hAD-MSC and hBM-MSC displayed much more rapid growth in RoosterBio media compared to a standard media formulation (Figure 1). In addition, hAD- and hBM-MSC displayed similar doubling time and expansion rates in RoosterBio High Performance Media.
hAD-MSC differentiate more rapidly towards adipose than hBM-MSC
RooterBio hAD-MSC and hBM-MSC are capable of differentiating to fat (adipogenesis) and bone (osteogenesis) (3,4). For adipogenesis, we assayed three lots of hAD-MSC (one RoosterBio and two ZenBio product lots) and, in parallel, two lots of hBM-MSC. Not surprisingly, hAD-MSC are highly adipogenic with abundant Oil Red O positive lipid vacuoles by day 8 or 9 of differentiation (Figure 2, left panels). hBM-MSC, in contrast, displayed less differentiation to fat compared to hAD-MSC at early and later time points (7). Lipid vacuoles were not present in cultures of hAD-MSC or hBM-MSC maintained in control (growth) media.
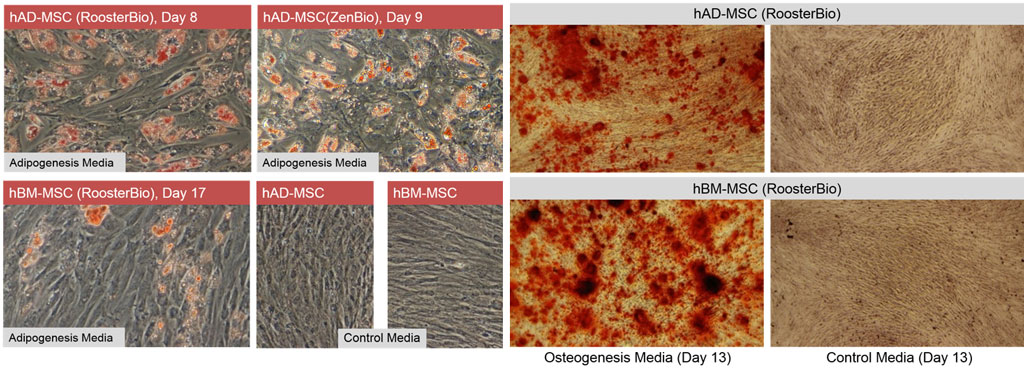
hAD- and hBM-MSC may display differences in osteogenic differentiation
Both hAD- and hBM-MSC undergo osteogenesis (Figure 2, right panels), as assessed by Alizarin Red staining. We noted qualitative differences in calcium deposition between our hBM-MSC and hAD-MSC donors, with hBM-MSC qualitatively being more efficient (7,8). However, quantitative analyses to confirm this were not performed at this time.
hAD-MSC donors consistently displayed more robust immunomodulatory activity than hBM-MSC donors
Immunomodulation is a key characteristic of isolated hMSC and is essential for their in vivo therapeutic roles(s) (2,7). In vitro,
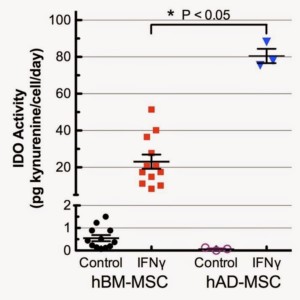
the immunosuppression potential of hMSC is assayed by IFN-γ activation of indoleamine 2,3-dioxygenase (IDO) and measuring kynurenine, an immunosuppressive product of IDO, in the cell culture supernatant (see here for more detail). The first interesting observation regarding IDO activity is that hAD-MSC had lower basal expression in unstimulated (growth media only) conditions when compared to hBM-MSC (Figure 3). IDO activity was highly upregulated in both hBM- and hAD-MSC by IFN-γ treatment. Of particular note, IDO activity in the presence of IFN-γ for hAD-MSC (n=3) was significantly higher for all 3 adipose donors (≈3.5 fold, p<0.05) than for all hBM-MSC manufacturing lots that we have manufactured to date and tested under identical conditions (Figure 4). This result is consistent with some, but not all, published studies (7,9,11,12).
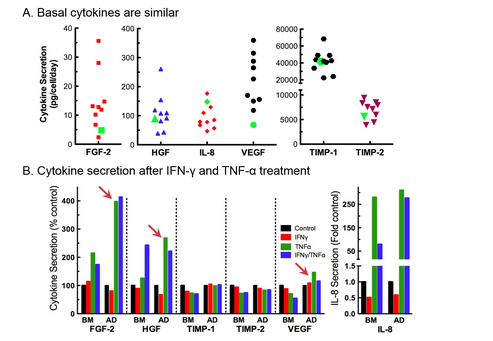
There are differences in cytokine secretion profiles of hAD-and hBM-MSC
As part of our standard QC assays, we measure and report secretion of angiogenic cytokines by each hMSC lot we manufacture. Unstimulated angiogenic cytokine secretion by hAD-MSC (green symbols, Figure 5A) is comparable to secretion levels of hBM-MSC (multiple lots, Figure 4A). We also measured angiogenic cytokine secretion profiles under priming conditions (unstimulated control, +IFN-γ, +TNF-α, +IFN-γ and TNF-α). Both hBM- and hAD-MSC responded to priming with inflammatory cytokines by altering their angiogenic secretion profiles (Figure 4B). Of particular note, differences in the secretion of FGF-2, HGF, and VEGF were noted between hBM- and hAD-MSC in response to TNF-α alone and in combination with IFN-γ (6,7).
Conclusions
hBM- and hAD-MSC share the minimal criteria for them to be classified as MSC. However, differences in hAD- and hBM-MSC functionality, in terms of their differentiation potential, immunomodulatory potential, and cytokine secretion profiles have recently begun to be published, and we find functional differences between these two tissue sources when cultured in our proprietary conditions as well. Our results underscore the need to characterize each population of hMSC, regardless of tissue source, and use their unique functional properties to choose the cell population best suited to each therapy or application. While the results presented here demonstrate differences between hAD- and hBM-MSC manufactured using RoosterBio expansion media and our protocols, it’s important to note that differences may, at least in part, be due to inherent donor-to-donor variability in addition to the tissue source. As we continue to add hAD-MSC donors and rigorously characterize them, we will update our blog with the results so that we may better understand differences between hMSC from a single tissue source and multiple tissue sources. Our primary objective is to continue enabling our customers to make informed decisions when it comes to their cell source.
References
- Mendicino M, Bailey AM, Wonnacott K, Puri RK, & Bauer SR (2014) MSC-based product characterization for clinical trials: an FDA perspective. Cell stem cell 14(2):141-145. http://www.ncbi.nlm.nih.gov/pubmed/24506881
- Murphy MB, Moncivais K, & Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & molecular medicine 45:e54. http://www.ncbi.nlm.nih.gov/pubmed/24232253
- Krampera M, Galipeau J, Shi Y, Tarte K, & Sensebe L (2013) Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15(9):1054-1061. http://www.ncbi.nlm.nih.gov/pubmed/23602578
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, & Gimble JM (2013) Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15(6):641-648. http://www.ncbi.nlm.nih.gov/pubmed/23570660
- Ranganath SH, Levy O, Inamdar MS, & Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell stem cell 10(3):244-258. http://www.ncbi.nlm.nih.gov/pubmed/22385653
- Strioga M, Viswanathan S, Darinskas A, Slaby O, & Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem cells and development 21(14):2724-2752. http://www.ncbi.nlm.nih.gov/pubmed/22468918
- Melief SM, Zwaginga JJ, Fibbe WE, & Roelofs H (2013) Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem cells translational medicine 2(6):455-463. http://www.ncbi.nlm.nih.gov/pubmed/23694810
- Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, & Kyurkchiev S (2008) Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell biology international 32(4):384-393. http://www.ncbi.nlm.nih.gov/pubmed/18262807
- Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, & Dilley RJ (2012) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem cells and development 21(12):2189-2203. http://www.ncbi.nlm.nih.gov/pubmed/22188562
- Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, & Kyurkchiev DS (2009) Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunology letters 126(1-2):37-42. http://www.ncbi.nlm.nih.gov/pubmed/19647021
- Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, & Blancher A (2005) Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. British journal of haematology 129(1):118-129. http://www.ncbi.nlm.nih.gov/pubmed/15801964
- Roemeling-van Rhijn M, Khairoun M, Korevaar SS, Lievers E, Leuning DG, Ijzermans JN, Betjes MG, Genever PG, van Kooten C, de Fijter HJ, Rabelink TJ, Baan CC, Weimar W, Roelofs H, Hoogduijn MJ, & Reinders ME (2013) Human Bone Marrow- and Adipose Tissue-derived Mesenchymal Stromal Cells are Immunosuppressive and in a Humanized Allograft Rejection Model. Journal of Stem Cell Research & therapy Suppl 6(1):20780. http://www.ncbi.nlm.nih.gov/pubmed/24672744
This blog was first published in Democratizing Cell Technologies, A RoosterBio Blog, – http://roosterbio.blogspot.com/2015/04/adipose-and-bone-marrow-derived-hmscs.html