Albumin Fatty Acid Profiles for cell culture media – Enabling Albumin Optimization for Cell Culture Media
Part 3 of the Cell Culture Media Optimization Series
A Guest Blog by Randall Alfano, Ph.D., Vice President Product Development, InVitria
Due to the multifaceted biological role of human serum albumin, inclusion in cell culture media has been shown to be extremely beneficial for the propagation of many cell types ex vivo. However, proteins isolated from human serum can have variable performance and potential adventitious agent contamination risks. Thus, the investigation into the inclusion of a recombinant alternative in serum free cell culture media is warranted. Due to the inherent complexity of albumin function, the successful substitution of human serum-derived albumin with recombinant versions in cell culture media can be somewhat involved. One particular function, fatty acid binding and subsequent delivery to proliferating cells, can have extremely profound effects in cell culture and is worthy of further discussion.
Fatty Acid Binding to Albumin
Fatty acids and their derivatives are a part of the larger and more loosely defined class of biomolecules known as lipids. Anatomically, these molecules consist of the hydrophilic carboxylic (-COOH) head and a hydrophobic hydrocarbon tail. This tail represents the primary differentiator amongst different fatty acids as the length and the number/position of carbon-carbon double bonds varies.
Due to their insolubility in aqueous solutions such as plasma, fatty acids require binding to transport proteins such as albumin. Albumin binding of these biomolecules has been extensively studied through the decades via modeling of organic dyes, anionic detergents, fluorinated/spin-labeled derivatives, and more recently by direct (13)C-labeled fatty acid binding to albumin (Spector 1975; Simard J 2006). Cumulatively, albumin contains at least seven independent intermediate to high affinity fatty acid binding sites that are spread throughout the molecule with an additional 20 low affinity binding sites (Vusse 2009). Studies utilizing NMR to detect (13)C-carboxyl labeled palmitate binding to human serum albumin was able to further delineate fatty acid preferential binding in that sites 2, 4, and 5 exhibited higher affinity while sites 1, 3, 6, and 7 exhibited lower fatty acid affinity (Simard J 2006). This difference in binding affinity is due to the shape of the binding sites in that the aliphatic chain of the fatty acid molecule is almost completely enclosed in higher affinity sites (Vusse 2009). Interestingly, these intermediate to high affinity sites are also known to overlap with binding sites for drugs and other endogenous compounds (Simard J 2006).
Fatty acids bind these albumin sites primarily by hydrophobic binding via the hydrocarbon groups that appear to extend along the entire fatty acid chain length. Indeed, primary association constants of binding increases with carbon chain length (Otagiri M 2009). Other modeling has indicated that electrostatic or hydrogen bonding interactions involving the carboxylate group also occur, albeit are less critical than hydrophobic interactions (Spector 1975). Cumulatively, albumin can carry up to a total amount of 1-2 molar equivalents of fatty acids (Ishima Y 2008).
Fatty Acid Delivery and Functions
In vivo, the primary fatty acid source arises from the digestion of dietary fats in the gastro-intestinal tract by catalytic pancreatic hydrolytic enzymes (Vusse 2009). Fatty acids are then transported to tissues via the lymphatic and vascular systems as either triacylglycerols or albumin-bound non-esterified fatty acids (Vusse 2009). Plasma concentration of fatty acids are typically around 5-10 nM (Vusse 2009). Delivery of fatty acids through the vascular endothelium to the underlying tissues for utilization is still a subject of study as multiple mechanisms have been proposed. However, experiments involving the transport of palmitate across rat heart endothelium demonstrated that fatty acids will dissociate from albumin and will subsequently be actively transported across the endothelial barrier (Tschubar F 1993). Further, Tschubar et al., determined that the major rate-governing step of fatty acid transport across cardiac endothelium was the dissociation of the fatty acid from albumin (Tschubar F 1993). Indeed, these studies are in agreement with other published works that the rate of fatty acid release from albumin is approximately 0.003 to 0.14 sec -1. (Scheider 1978; Scheider 1979). Trans endothelial fatty acid delivery has been proposed to be facilitated by both endothelial cell luminal surface proteins, such as albondin, as well as endothelial cytoplasmic fatty acid binding proteins (cFAPB) (Vusse 2009).
Once in the target cell, fatty acids have multiple functions across a plethora of biological structures and physiological and pathophysiological processes. Perhaps the most critical function is to provide structural elements to cell membranes and mitochondria (Wiktorowska-Owczarek A 2015). Saturated fatty acids, with the absence of any carbon-carbon double bonds within the hydrophobic fatty acid tail, are able to densely pack and create rigid membrane structures. Unsaturated fatty acids, conversely, cause a ‘tail’/hydrocarbon chain bending which greatly enhances membrane elasticity and fluidity (Wiktorowska-Owczarek A 2015). Further, the n-3 and n-6 polyunsaturated fatty acids, such as linoleic and linolenic acid, are precursors for the synthesis of eicosanoid hormones such as prostaglandins (Grammatikos S 1994; Wiktorowska-Owczarek A 2015). Fatty acids have also been shown to activate specific enzymes such as phospholipases which may activate protein kinases in signaling transduction that modulate different cellular responses (Gillham H 1996).
Serum Derived and Recombinant Albumin Fatty Acid Profiles
Given the broad functionality of fatty acids in multiple biological processes, it would be expected that the inclusion in cell culture media would be beneficial for most mammalian cell types. However, the response is variable depending on the cell type, levels, and types of fatty acid inclusion (Butler M 1998). A murine hybridoma grown in the presence of 50 µM linoleic acid-supplemented serum free media demonstrated significantly higher survival than control cells in agitated cultures (Butler M 1998). However, the same fatty acid was found to dose dependently and significantly inhibit in vitro cumulus cell expansion and delay the development of bovine oocytes to the metaphase II stage (Marei W 2010).
Given these conflicting observations, it would be ideal to optimize fatty acid supplementation in any cell culture media application by individually screening each fatty acid. However, this approach would prove to be time consuming and logistically impossible as most commercially available albumins include some level of fatty acid conjugated to the albumin protein already. Therefore, in order to better understand the lipid profiles of commercially available serum-derived and recombinant albumins that were previously analyzed for analytical properties, we examined fatty acid levels in each of these albumin products.
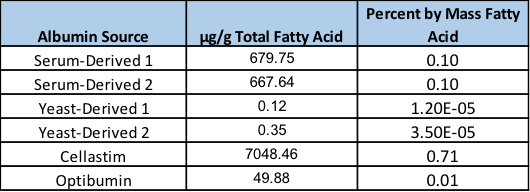
This analysis revealed extremely variable levels of fatty acids among the different albumin products (Figure 1). Interestingly, the serum derived albumins obtained from two independent vendors exhibited almost identical levels of fatty acids at 679.75 and 667.64 µg/gram of albumin, respectively, corresponding to approximately 0.1% of the total mass. However, the yeast-derived recombinant versions exhibited extremely low fatty acids at 0.12 and 0.35 µg/gram of albumin translating to approximately 8,000 and 2,800-fold lower fatty acid levels by mass in comparison to the serum-derived products. Conversely, Cellastim contains approximately 10-fold higher fatty acid loads compared to the serum-derived albumins with roughly 7 mg fatty acids per gram of albumin. Optibumin was noted to contain approximately 10-fold lower lipids than the serum-derived albumins deriving an albumin product with 0.01% lipid mass.
A detailed look into which fatty acids are present on each albumin molecule is warranted to further investigate such a large
dissimilarity of total fatty acids amongst the different albumin products. Levels of each fatty acid were normalized to the percent of the average of the serum-derived albumins and presented in Figure 2. As mentioned, the serum-derived albumins exhibited very similar total lipid loads. Upon closer inspection, very similar levels of each fatty acid were observed in these albumin preparations from different vendors. However, a comparison to the recombinant versions determined a strikingly different profile in both specific species present and loads of each fatty acid. The only similarity noted amongst all the albumins was that Docosapentaenocic -omega 3, Lignoceric, Myristoleic, and Lauric acids were found not to be present in any measurable amount.

The yeast-derived recombinant albumins exhibited somewhat similar profiles to serum-derived albumin in that fatty acids not present on serum-derived albumin were also not present on the recombinant versions. However, these yeast-derived recombinant albumins lacked 11 different fatty acids that were detectable on both serum-derived albumins. Further, fatty acids that were present on the yeast-derived albumins exhibited a 400-18,000-fold reduction when compared to that of serum-derived albumin. Each of these lipid species that were found to be reduced in the yeast-derived albumins provides further evidence for the low fatty acid percent mass in these recombinant albumins.
The recombinant albumins Cellastim and Optibumin have a markedly different profile when compared to serum-derived albumins. Cellastim and Optibumin were shown to lack Pentadecanoic, Vaccenic, Arachidic, Eicosadienoic, Docosahexaenoic acids that are present at some detectable level on serum-derived albumins. Further, 11-Eicoenoic, Dihomogamma-linolenic and Arachidonic acids could not be detected on Cellastim while Optibumin was negative for Eicosapentaenoic, Palmitoleic, Eicosapentaenoic, and Behenic acids. In addition, Cellastim was found to have high levels of gamma-linolenic, Erucic, Docosatetraenoic, and Docosapentaenoic -omega 6 fatty acids that were not detectable on serum-derived albumin. Similarly, Optibumin possessed some measurable quantities of Meads and Nervonic acids.
Fatty acids that were found to be present on both serum-derived albumin and Cellastim were noted to be extremely variable and no particular ratio or pattern of increase to account for the approximately 10-fold higher lipid load in Cellastim could be identified. Myristic acid was found to be approximately 6-fold higher loads in Cellastim while serum-derived albumin was found to harbor 3-fold higher levels of Stearic acid. Optibumin, however, proved to be slightly more predictable in that fatty acid species present on both albumin types exhibited approximately a 6-40-fold reduced level in Optibumin when compared to serum-derived albumin.
Conclusions and Final Remarks
Taken together, albumins derived from human serum exhibit a significantly different fatty acid profile when compared to any of the recombinant versions. However, like the analytical differences previously determined, albumin fatty acid profiles may be suggestive but are not ultimately indicative of albumin function in vitro. Thus to this point, only analytical and biophysical differences have been noted among the different commercially available albumin sources in the context that they simply exist and that conclusion of albumin equivalence is erroneous.
Therefore, given that the biophysical differences via monomer purity, contaminating substances, and now lipid profiles have been established among the different albumin proteins, it is critical to explore how these analytical qualities translates to functionality via the interrogation in multiple industry and research-relevant cell systems. Further, the exploration of specific manipulation of lipid profiles to improve albumin functionality in vitro will be explored.
Don’t miss Part I and Part II of the Cell Culture Media Optimization Series –
“Generating Recombinant Versions of Human Serum-Derived Proteins – Transferrin and Albumin”
“Albumin in Cell Culture Media – An examination of quality and function“
References
Butler M, H. N., Barnabe N, Gray T, Bajno L (1998). “Linoleic acid improves the robustness of cells in agitated cultures.” Cytotechnology 30: 27-36.
Gillham H, B. K. (1996). “31P NMR measurments of the effects of unsaturated fatty acids on cellular phopholipid metabolism.” Magnetic resonance in Medicine 35: 481-488.
Grammatikos S, S. P., Victor T, Miller W (1994). “Diverse effects of essential (n-6 and n-3) fatty acids on cultured cells.” Cytotechnology 15: 31-50.
Ishima Y, A. T., Kragh-Hansen U, Hiroyama S, Sawa T, Suenaga A, Maruyama T, Kai T, Otagiri M (2008). “S-nitrosylated human serum albumin-mediated cytoprotective activity is enhanced by fatty acid binding.” JBC 283(50): 34966-34975.
Marei W, W. D., Fouladi-Nashta A (2010). “Impact of linoleic acid on bovine oocyte maturation and embryo development ” Reproduction 139(6): 979-988.
Otagiri M, C. V. (2009). “Pharmaceutically important pre- and posttranslational modifications on human serum albumin.” Biol Pharm Bull 32(4): 527-534.
Scheider, W. (1978). “Dissociation rate of serum albumin-fatty acid complex from stop-flow dielectric study of ligand exchange.” Biophys J 24: 260-262.
Scheider, W. (1979). “The rate of access to the organic ligand-binding region of serum albumin is entropy controlled.” Proc Natl Acad Sci USA 76: 2283-2287.
Simard J, Z. P., Hamilton J, Curry S (2006). “Location of high and low affinity fatty acid binding sites on human serum albumin revealed by NMR drug-competition analysis.” J Mol Biol 11(361): 336-351.
Spector, A. (1975). “Fatty acid binding to plasma albumin.” J Lipid Res 16(3): 165-179.
Tschubar F, R. H., Kammermeier H (1993). “Fatty acid transfer across the myocardial capillary wall.” J Mol Cell 25: 355-366.
Vusse, v. d. (2009). “Albumin as a fatty acid transporter.” Drug Metab Pharmacokinet 24(4): 300-307.
Wiktorowska-Owczarek A, B. M., Nowak J (2015). “PUFAs: Structures, Metabolism and Functions.” Adv Clin Exp Med 24(6): 931-941.