
The Importance of Resin Selection in Development of a Platform Bioprocess Film
Utilizing single-use technologies for biomanufacturing is growing due to several operational benefits including; reduced cleaning and validation, decreased water usage, lower initial capital investment, increased manufacturing flexibility, and faster turnaround time between runs.
As the implementation of single-use products has grown, so too has the expectation for improved performance and an increase in the number of application areas involved. Issues have arisen with respect to container integrity, bag flexibility and extractable/leachable profiles. For example, end users want to see single-use products provide the strength needed to maintain container integrity, while at the same time provide the flexibility required for use in applications like bulk liquid transfer and rocking bioreactors. In addition, it is critical that the single-use products do not leach any compounds into the culture that would have a negative impact on the cells or the final product.
In developing single-use films that are specifically designed for biomanufacturing, the material used for construction is key and requires a great deal of research and testing. Single-use containers are constructed from plastic films, which are often composed of several layers of polymers with additives for processing and performance. The flexural properties of these layers are important to performance.
In addition, the diversity of applications for single-use containers requires film that can achieve a wide variety of performance attributes such as mechanical strength, flexibility, biocompatibility, and suitable gas barrier properties to name a few. Thus, the right balance of chemical composition and film architecture is critical for achieving desired performance across many applications.
GE Healthcare and Sealed Air Corporation recently presented on this topic in their poster titled, “Resin selection to optimize the flexural strength of bioprocess film”. In the poster they described GE Healthcare’s, Fortem™, single-use bioprocess platform film. Fortem will be used in GE’s entire portfolio of single-use products. This poster focuses on how GE Healthcare selected the resin and created the architecture for their new bioprocess film and how these can be optimized to maintain critical performance attributes, such as container integrity and gas barrier properties, under the significant forces exerted during operations such as bulk liquid transportation and WAVE Bioreactor™ rocking.
Poster Highlights
Film Structure and Construction Materials
In the poster, authors provide a look at the Fortem 10 layer film structure (Figure 1) including the materials selected for each layer.
Figure 1:
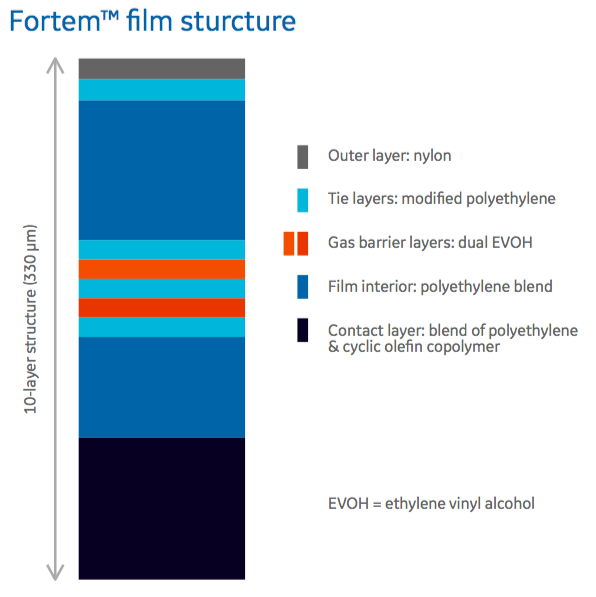
It was important that each layer be comprised of material that met the needs for that layer. In the poster, the authors describe the unique properties of each layer.
- The fluid contact layer, polyethylene and cyclic olefin copolymer (COC) is compliant with EP 3.1.3, EP 3.1.5, JP 7.02, USP Class VI and also COC acts as a macromolecular slip agent, eliminating the need for traditional small molecule additives.
- The gas barrier has two different types of EVOH (alcohol substitution) incorporated into the structure that provides a barrier to gases in both wet and dry conditions.
- The outer layer is comprised of a specific nylon chosen to provide strength even in humid conditions. The interior layers are composed of a polyethylene blend for robustness and flexibility over a wide temperature range.
- There is also an antioxidant package, which is a selection of additives and placement in film optimized for minimal impact on mammalian cell culture performance.
Creating the right film architecture
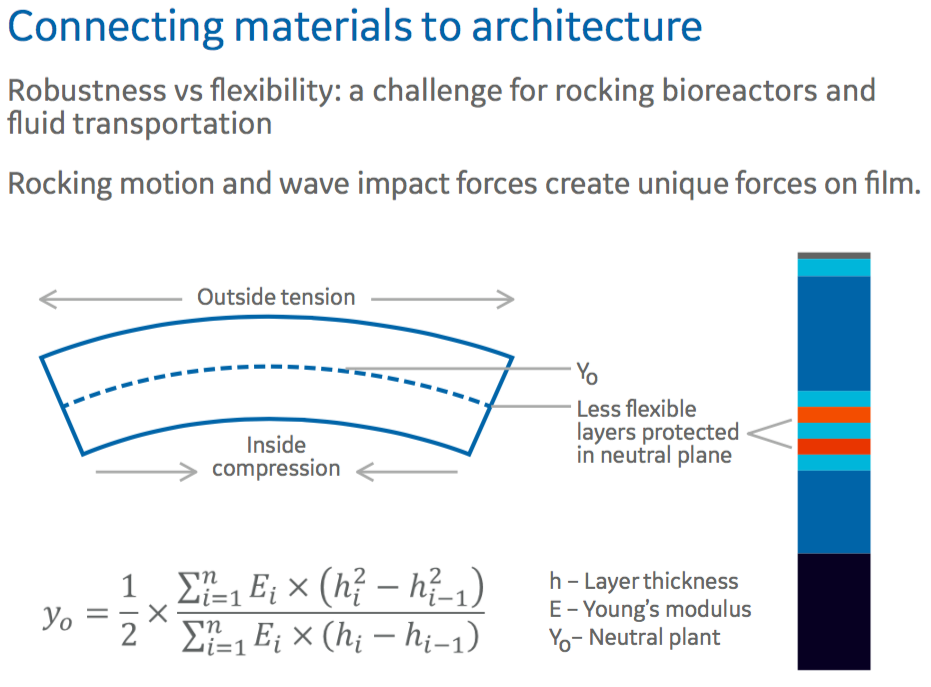
Authors then described how they utilized these materials in creating the architecture of the film to address specific needs. Primarily, how they balanced the need for strength with flexibility with respect to rocking bioreactors that impose unique forces on film. There must be the right balance of robustness and flexibility (Figure 2).
Figure 2:
Film Impact on Cell Culture
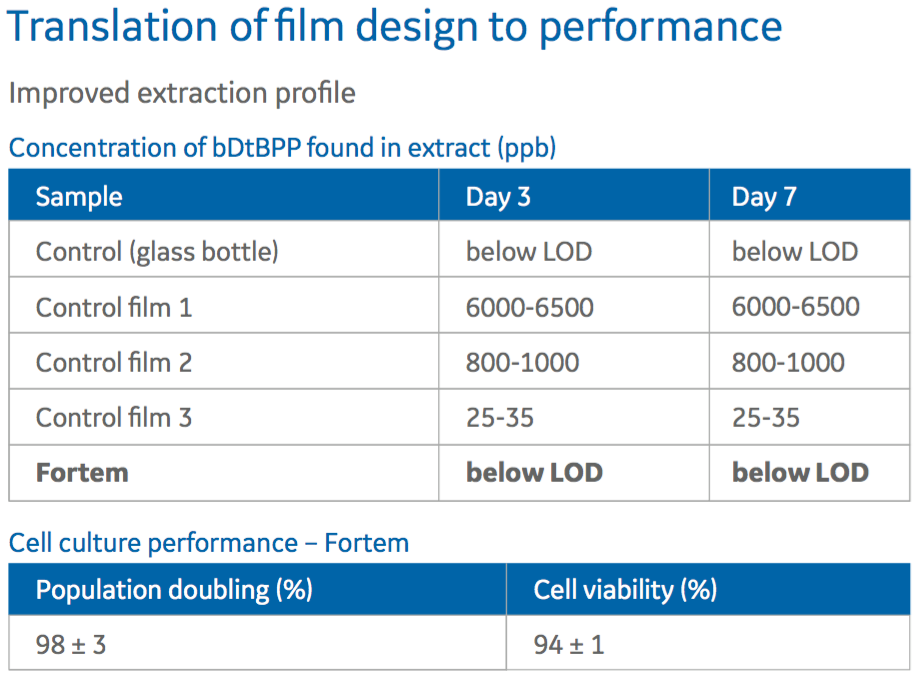
Authors also examine the effect of Fortem on cell culture performance by examining the extraction profile. Specifically they looked at the concentration of bDtBPP found in extract at ppb. What they found was that Fortem film provided concentrations of bDtBPP at levels below LOD, like the glass bottle control (Figure 3).
Figure 3:
Summary
The authors describe that to keep pace with the needs of the biomanufacturing industry, the films used in single-use bioprocess technology should be purposefully designed for bioprocess applications. With the new Fortem film, the combination of resin selection and film architecture has been carefully selected and skillfully optimized to deliver innovation.