
Efficient single cell isolation and proof of clonality in one step
Cell line development is a crucial part of biomanufacturing and sets the stage for future success by enabling the discovery of high yield and high quality clones.
Within the cell line development workflow, one of the most critical steps is single cell isolation. The goal is to culture single cells in individual wells, which is necessary to ensure clonality. The FDA requires proof of clonality and companies must provide evidence that each cell line used in manufacturing has been generated from a single cell. This verification typically involves a combination of statistics and images to provide the required evidence.
Single Cell Isolation Challenges – Things to consider
Each method for cell cloning has its advantages and limitations. When evaluating the best method to use, it is important to consider the key challenges.
Empty wells, multiple cells, and dead cells
Of course anything other than one cell in a well is a waste of personnel time, consumables, and resources. However, getting just one live cell in a well can be more challenging than it sounds. Anyone who has worked in this area is familiar with a plate that has a mix of well results. For example, the low seeding density used in limited dilution means there will be a high number of empty wells. With flow-cytometry most wells will contain a cell but the depositing technique can stress cells and can result in several wells containing dead cells.
Resources – Time and Personnel
Cell line development frequently requires tight turnaround deadlines, these pressures necessitate smart choices when deciding which single cell isolation method to use. Some methods are more time consuming or efficient than others. Limited dilution and flow cytometry, for example, are both very time consuming isolation techniques and cause further effort downstream due to inefficiencies.
Required throughput is also an important consideration. With more labor intensive or less efficient cloning methods, it may not be possible to screen enough clones to find a suitable option. Obviously the more clones you are able to screen, the more chance you have of finding a great one.
Proof of clonality
As discussed, documenting clonality is a critical part of the regulatory submission process. Some single cell isolation methods require more extensive statistical analysis. Statistical analysis tends to be more time consuming and can be less straightforward than using images with more opportunity for errors in the documentation process. Selecting a method that will provide the most sure and efficient way to establish clonality is vital to a more straightforward regulatory package later that assures monoclonality.
Single cell printing
In an effort to create a more efficient method for single cell isolation and clonality documentation, new technologies have been introduced. One of the new technologies is CloneSelect™ Single Cell Printer developed by Cytena and Molecular Devices. The printer uses microfluidics-based technology (similar to an ink jet printer) that sorts and deposits single cells into standard 96- or 384-well microplates. This provides high efficiency and high viability while simultaneously providing image evidence of monoclonality.
How it works
The CloneSelect Single Cell Printer uses a disposable cartridge to deposit single cells into a well. Individual cells derived from a cell suspension are encapsulated in aqueous droplets. The cell suspension is released in droplets. While the droplet is moving through the functional part of the cartridge, a series of five pictures per well is taken. These pictures provide an image trail for proof of clonality. Specialized software uses the images to identify whether a drop contains a single cell. If it is determined that a drop contains none or more than one cell, the droplet is vacuumed away and does not get deposited in the well. Thus only single cells should be present in the wells. Figure 1 provides an illustration of the droplet seeding process.
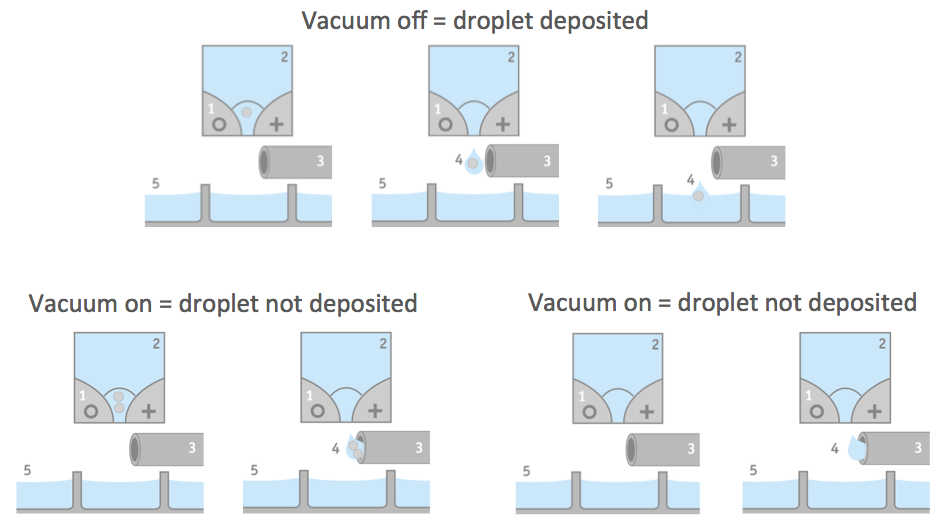
Efficiency
The CloneSelect SCP is unique because it takes an image prior to depositing the droplet into the well. The software can then make decisions about whether to deposit the droplet or not. This results in higher efficiencies and a higher percentage of wells containing only one cell. Data provided by Molecular Devices, shows an average cell deposit efficiency of 80% and an average cell viability of 75% when using the CloneSelect SCP (Figure 2)
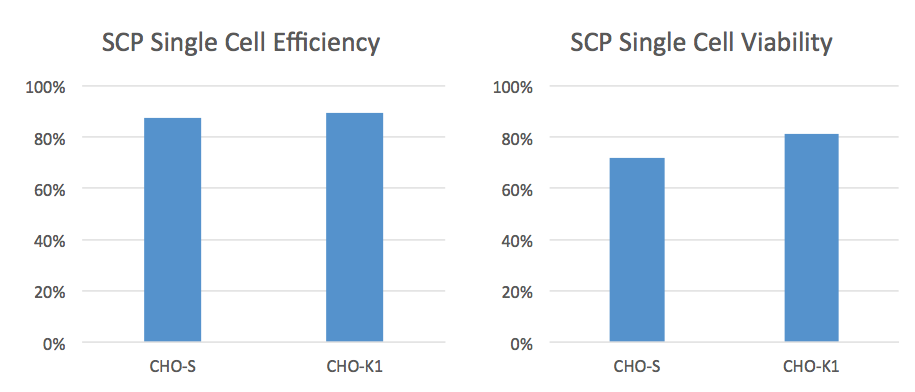
Another nice feature is the disposable microfluidic cartridge that permits simple cell prep with one dilution and since it is single-use, also prevents cross contamination.
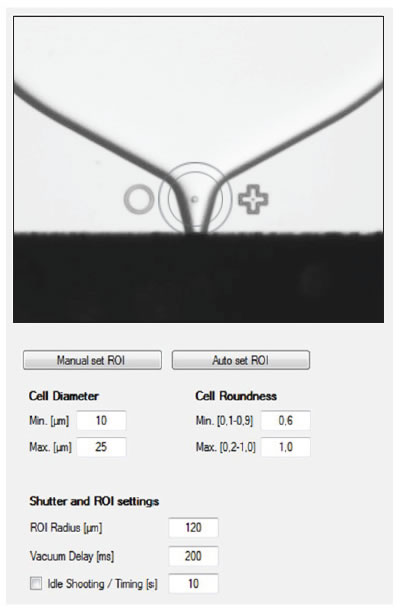
Screening Capabilities
Another unique feature is that the imaging system can also be used to screen for cell size and shape prior to deposit, thus permitting the removal of any cells that appear to be in poor health. This additional screening option improves the overall cell viability for the plate. Figure 3 shows an image of the CloneSelect SCP in progress and the simplified software used to enable screening by cell parameters.
Workflow
The CloneSelect SCP integrates nicely into the cell line development workflow. The product can be combined with imagers that evaluate the growth rate of clones or microplate readers that look at protein production. It also has a small footprint and can easily fit inside a BSL2 hood for further sterility.
It deposits single cells into a 96 well plate in 5-10 minutes and has a throughput of ~1,000 clones. If higher throughput is required, there are other products such as the ClonePix™ that can accommodate a significantly higher throughput of ~ 10,000 clones.
For more information, please see moleculardevices.com/cloneselectSCP