
How Single-use Systems are Improving Bioprocess Development
A Guest Blog by William Whitford, Sr. Marketing Manager, Cell Culture, Thermo Scientific Cell Culture & Bioprocessing
There are a number of pressures to improve the power, efficiency and economy of process development in culture based biopharmaceutical manufacturing. Such new regulatory initiatives as QbD are demanding more flexibility for designing greater process robustness and Design Space definition. New product categories, such as biosimilars, are demanding greater speed and economy in process development (1). Single use product contact materials and the systems employing them have become a standard in bioprocess development (2).
Biopharmaceutical sponsors not only have single-use technologies to support many individual operations, but are increasingly presented with a variety of entire systems employing single-use and disposable product contact surfaces. Such systems support a growing number of manufacturing operations- beginning upstream with disposable mixing, transfer and filtering for process fluid and culture media preparation. Single-use bioreactors (SUBs) and Single-use Mixers (SUMs) allow for disposables-based capability in cGMP and ISO certified manufacturing up to the 2,000-L scale and (Fig. 1). Downstream, single-use mixing, filtration, centrifugation and chromatographic systems are in various levels of acceptance, and offerings now support such operations as viral clearance, drug substance storage, and formulation/fill (3).
Employing Single-use Systems (SUS) in the process development (PD) of approved products provides a number of benefits, from speed and efficiency to cost savings to heightened safety (4). These characteristics and operational values have been well explained and defined (5). Efficiency is supplied by greatly increased operational flexibility, reduced process turnaround time, ease and safety in product changeover, and economy of site-to-site transfer or replication. Cost savings include reduction in capital requirements and process footprint and in the elimination of not only entire operations (such as cleaning) but also their associated service and validation procedures. SUSs offer new advantages in PD and operations while maintaining many of the values of the classical materials they replace.
Process Development Economy
The greatly reduced production suit footprint required by SUBs and SUMs reduces facility design, construction and consequent costs (Fig. 2). Savings also come from reduced service requirements, such as the severely reduced (and sometimes eliminated) requirement for regulated water or steam, due to the elimination of CIP and SIP systems. Manufacturing modeling programs reveal that total manufacturing costs using SUS can be significantly less than that from conventional systems. Because of the reduced capitalized cost of the smaller facilities and equipment with SUS, initial charges are dramatically reduced. Also, costs from the disposable elements of the SUS are deferred to the time of use, converting fixed to variable costs. When increasing production, SUS provide an easy and inexpensive “scaling out” of capacity.
Process Development Efficiency
The utility of SUTs have been demonstrated for all major animal cell-lines and production modes, from CHO to insect cells, in fed-batch or perfusion production modes. This allows employing the precise format determined to yield the most efficient production.
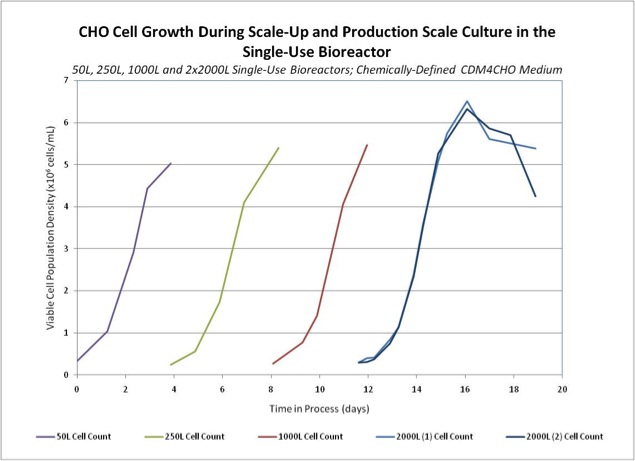
Because they do not require CIP and SIP, SUS accelerate facility design and construction schedules. Reduced facility requirements can even allow installation in existing suits– saving many months of time. Decreased or eliminated services also greatly shorten the time spent in facility, equipment and process qualification and validations. The reusable non-product contact hardware for such systems as single use mixers (SUMs) and single use bioreactors (SUBs) can be constructed and installed in a matter of weeks, remarkably reducing equipment design and build times. The remarkable scalability of some SUS equipment is a great advantage in PD and technical transfer (Fig. 3). The efficiency of custom designed, multiply overwrapped, freezable and pre-sterilized flexible bioprocess containers (BPCs) eases GMP cold chain and good storage practice procedure establishment and validation.
There are a quite a number of “flexibilities” provided by SUTs that can rather spectacularly reduce process development time. Many SUSs support flexibility in the designation of integral components. From operating systems to connectors the client is able to select the particular components and vendors desired– reducing cost and time in component qualification. Component and flowpath specifications are easily altered after initial design, supporting easy and rapid response to updates in equipment, components, or flow. Development runs can be accelerated because of the reduced turnaround time with SUBs and SUMs (often a matter of hours). Many SUS skids can be easily re-located, even wheeled, to new locations within plant, opening doors of opportunity and shortening PD time. Finally, SUS support the employment of divergent raw material and products types and classification, accelerating the design of trial runs.
So we are currently seeing a revolution in bioprocessing with the uncharacteristically rapid adoption of single-use materials throughout the production train. But what’s even more exciting are the newer SUT and products in the pipeline supporting such intriguing prospects as completely closed and continuous manufacturing.
To learn more about large scale bioprocessing solutions in an easy and fun format, you may visit www.youtube.com/bioprocessing
Figure 1 . SUS offerings profide choices to various sponsors needs.
 |
1A A “turnkey” single-use bioreactor providing maximum speed and convenience of establishing de novo bioproduction capability. (Courtesy Thermo Fisher Scientific) |
 |
1B An “open-architecture” single-use bioreactor providing maximum SUT flexibility and economy. (Courtesy Thermo Fisher Scientific) |

Footnotes
-
1. Whitford, WG. Single-Use Technology Supports Follow-On Biologics. BioProcess Int . 10(2) 2012: s20–s31.
-
2. Whitford WG. Single Use Systems as Principal Components in Bioproduction. BioProcess Int. 8(11) 2010: 34–42.
-
3. Laukel M, et al. Disposable Downstream Processing for Clinical Manufacturing. BioProcess Int.9(5) 2011: s14–s21.
-
4. Langer ES. CMOs Ratchet up use of Single-Use Devices to Improve Performance. BioPharm Int. 24(11) 2011: 27-30.
-
5. Eibl R, Eibl D. Chapter 18. Practical Aspects of Establishing Pharmaceutical Recombinant Proteins from Research to Development in Disposable Bioreactors, In: Single-Use Technology in Biopharmaceutical Manufacture, Editor(s): Regine Eibl, Dieter Eibl. Published Online: 19 JAN 2011.