Antibody Labeling for Flow Cytometry
A guest blog by Klaudyna Schmidt, Innova Biosciences
Introduction
With respect to cellular analysis, the underlying principle of flow cytometry is that a cell suspension is focused into a single cell stream, which passes through a light source (typically a laser beam). The scattered and emitted fluorescent light (if the cells are fluorescently labeled) is subsequently measured using a range of detectors and these measurements are used to generate multi-parameter data sets that describe the physical characteristics of the cells and their fluorescent properties. The size and granularity of cells can be identified on the basis of their forward and side light scatter characteristics (FSc and SSc respectively). Their characteristics and/or expression of different proteins can be further defined by pre-staining with fluorescently-labeled antibodies or molecules that identify cellular components and / or integrity (viability) or, indeed, fluorescently-labeled proteins.
Flow cytometers can evaluate cells at an extremely rapid rate (up to several tens of thousands of events (cells) per second) and can detect as few as 500 molecules per cell. The speed and sensitivity of flow cytometry therefore makes it ideally suited to the analysis of minor cellular populations. For general information on flow cytometry, please see our webinar “A Beginner’s Guide to Flow Cytometry.”
Reagent Sourcing and Availability
The number of products and suppliers of reagents for flow cytometry is rapidly increasing and it is becoming progressively more difficult to identify the appropriate combination of reagents for a particular flow cytometry experiment. Furthermore, it is not always the case that the labeled antibody that is required will be available, thus approaches that allow flow cytometry users to generate their own panel of labelled antibodies are needed.
Antibody Labeling Kits
One solution, which allows users to generate their own panel of labelled antibodies, is the use of antibody labelling kits. The simplification of the antibody labeling process (most notably the elimination of column separation steps) circumvents many issues that have beset traditional procedures for years, i.e. loss of material, sample dilution during column chromatography, and batch-to-batch variation.
Lightning-Link®is an antibody labeling kit, which provides an easy to follow, simple to use process for flow cytometry users to label their own antibodies. The Lightning-Link® process is summarized in the figure below. The purified antibody to be labeled is transferred into a vial of lyophilized mixture containing the label of interest. Dissolution of the vial contents activates the chemicals that mediate the antibody labeling reaction. As there are no purification or separation steps (byproducts of the reaction are completely benign), antibody recovery is close to 100%. The simplicity of the approach means that the labeling procedure can be completed in less than thirty seconds. A timed demonstration of the antibody labeling process can be seen here (Lightning-Link video). The labeling chemistry involves the free amine groups found on lysine amino acids, and so any antibody or protein, irrespective of isotype or species, can be labeled.
Although the antibody labeling procedure is very simple, the chemical approach is sophisticated – allowing the formation only of antibody-label conjugates, in a gentle and controlled process.
Advantages of Antibody Labeling Kits
These simple antibody labeling kits are of special interest to the flow cytometry community, as there are over 40 different labels including fluorescent proteins, dyes and tandems, which span the whole light spectrum from UV to far infrared.
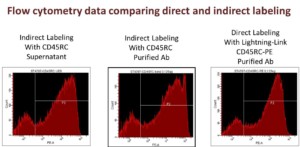
It is therefore now possible for the flow cytometry user to design and develop a unique set of labeled antibodies for use in the flow cytometer using commercially sourced unlabeled antibodies. Furthermore, there are many benefits associated with direct labeling of a primary antibody:
- Eliminates the need for the use of secondary antibodies, thus reducing the number of incubation and wash steps – saves both time and money.
- In experiments which use several antibodies simultaneously, cross-species reactivity is not an issue as the primary antibodies can be labelled with different dyes. With indirect detection, cross-species reactivity of secondary antibodies is often a problem.
Additional information can be read in the “Guide to Antibody Labeling and Direct Detection.”
Of special interest – there are now over 250 published references which cite the Lightning-Link® kits for a variety of applications including flow cytometry. The references can be viewed at www.innovabiosciences.com