Application of the Roche Cedex Bio Analyzer in the Culture of Human Pancreatic Islets and Adult Human Mesenchymal Stem Cells
A Guest Blog by: David W. Scharp, MD
Prodo Laboratories, Inc. and Scharp Technologies, Inc.
Introduction
While the Cedex Bio Analyzer was designed for production monitoring of nutrients, electrolytes, metabolites, and other testing, there are other applications that can benefit from these important test results outside of biopharmaceutical manufacturing. This report presents preliminary results using the Cedex Bio Analyzer for two very different applications of primary cell cultures: human islets under long-term culture in Prodo Laboratories (1, 2) and adult human mesenchymal stem cells producing new peptides for skin care projects in Scharp Technologies (3). Understanding the value in these two applications suggests there are other non-fermentation cell culture applications that can benefit from this type of tissue culture monitoring.
The Cedex Bio Analyzer is an efficient, medium-throughput analyzer that currently permits the rapid measurement of sodium and potassium through one module (ion-sensitive electrode), and nutrients and metabolites such as glucose, lactate, ammonia, LDH, glutamine, glutamate, IgG, and acetate through the second module (photometric analysis). Upcoming additions to this list as new measurements include iron, calcium, magnesium, and phosphate as well as pyruvate, total protein, and glycerol. Because of its small footprint and ease of testing, the Cedex Bio Analyzer is ideal for applications outside of fermentation that can benefit from routine monitoring to ensure stable cell culture conditions. It also has significant benefit in the development of new culture media and conditions as well as for GMP production of different kinds of cells for research and transplantation.
Human Islet Culture Testing
With the exponential expansion of clinical diabetes in the world today, there is significant pressure for investigators and distributors of human islets to provide a more standardized and uniform quality of human islets for research and new drug candidate testing. Our efforts at Prodo Laboratories have recently turned to evaluating the ability to provide successful long-term culture of human islets using PIM(R)® -“Recovery” and PIM(S)® – “Articles” culture media (Prodo Islet Medium, Prodo Laboratories, Inc.) to extend successful and functional survival of adult human islets to 4 weeks of in vitro culture. The ability to maintain human islet function for this period of time can clearly benefit research by permitting in vitro islet survival for kinetic studies, which simply cannot be accomplished with only a few days of islet culture. The ability to achieve 4 weeks of functional cultured islet survival can also permit performing only a single islet transplant using two clinical islet preparations rather than having to perform two separate transplant procedures. While insulin and glucagon staining was seen out to 4 weeks in the islets cultured in both the PIM(R)® culture group and the CMRL+HABS (Connaught Medical Research Laboratories medium + human AB serum) culture group, the CMRL+HuSA (HuSA – human serum albumin) group showed significant islet disintegration and loss of islet cell integrity by histology.
Functional islet survival as assessed by Glucose Stimulated Insulin Release was highest in the PIM(R)® group, ranging from 3.5 (insulin ng/ng DNA) to 2.5 (insulin ng/ng DNA) in the first 2 weeks, and remaining at 1.5 (insulin ng/ng DNA) for weeks 3 and 4. Islets cultured in CMRL+HABS had only 1.6, 0.6, 0.4, and 0.6 total insulin released (insulin ng/ng DNA) at weeks 1 to 4, respectively, and islets cultured in CMRL+HuSA had only 2.5, 0.9, 0.2, and 0.1 total insulin released (insulin ng/ng DNA) at weeks 1 to 4, respectively.
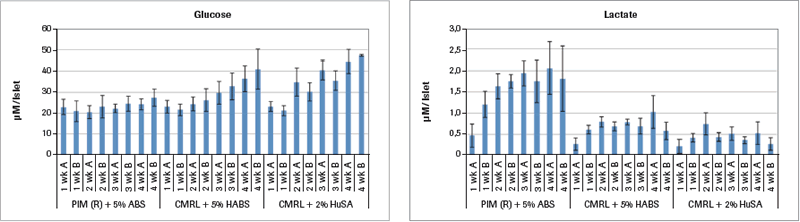
Analysis of samples run on the Cedex Bio Analyzer demonstrates some reasons for the inferior results from the two CMRL-based culture media as shown in Figure 1A.
Two Cedex Bio Analyzer samples were taken for each of the 4 weeks of the study that are shown in Figure 1A (mean value ± SEM). The glucose values for PIM(R)® remain stable throughout the 4 weeks. But in both the CMRL+HABS and the CMRL+HuSA groups, the glucose values continuously rise, with the largest rise in the CMRL+HuSA group throughout the 4 weeks. Since the glucose values in each of the media are the same with each medium change, the only explanation for this rise in glucose is that the islets in these last two groups are simply not metabolizing the glucose as well compared to the PIM(R)® group, which is found utilizing glucose.
This is confirmed by analysis of the lactate concentrations shown in Figure 1A. For the PIM(R)® group, the starting lactate level of 0.5 µM lactate per islet equivalent (IEQ) rises to a high of 2.0 µM lactate per IEQ in week 4. Glucose levels maintain throughout the 4 weeks, representing glucose metabolism. The lactate levels for both the CMRL+HABS group and the CMRL+HuSA group never rise above the 0.5 µM lactate per IEQ throughout the 4 weeks, indicating very little metabolism in these two groups that have much poorer insulin release in response to glucose challenge.
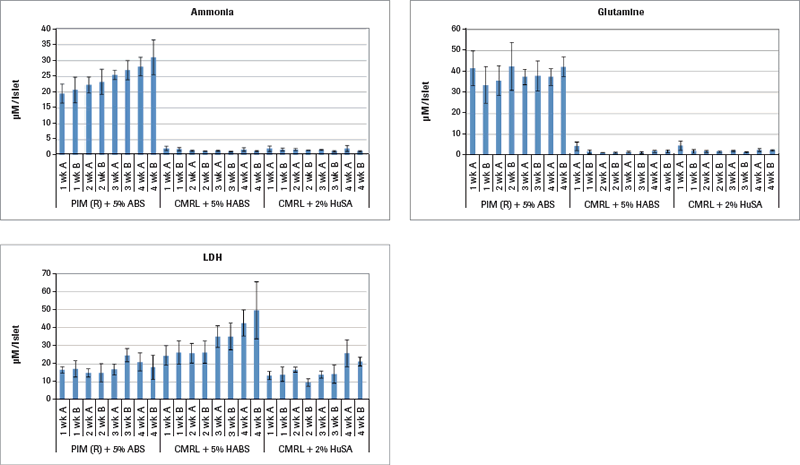
Continuing the metabolism analysis in Figure 1B, results demonstrate that only the islets cultured in PIM(R)® are metabolically active, as that is the only group producing ammonia. The other two culture media groups limited the islet metabolism to the extent that they failed to produce any ammonia. Glutamine is an important nutrient additive to the PIM(R)® culture medium as a cellular energy source like glucose involved in protein synthesis and other cellular functions. Ammonia is also an important metabolite from glutamine. Lactate dehydrogenase (LDH) in cell culture is contained within living cells. Release of LDH in the medium is one sign of cell death. The PIM(R)® cultured islets start their culture with 15.000 µM LDH per IEQ and reach a maximum of 20.000 µM LDH in weeks 3 and 4. In contrast, the CMRL+HABS cultured islets start at 18.000 µM LDH and rise to nearly 50.000 µM LDH, signaling significant cell death. Interestingly, the CMRL+HSA cultured islets did not show levels higher than 25.000 µM LDH throughout, while they were clearly not metabolizing. There is an observable difference in the histology between these three groups of 4-week cultured islets. The PIM(R)® group maintained large islets throughout the time course. The CMRL+HABS group also maintained intact islets throughout. However, the CMRL+HuSA group disintegrated into islet fragments and single cells with time of culture. Perhaps their release into very small islet aggregates that were not metabolizing kept them more alive than the CMRL+HABS group that was not metabolizing but remained in larger, intact islet aggregates. The utilization of the Cedex Bio Analyzer clearly provides more insight into the metabolic state of islets during long-term culture out to 4 weeks.
Adult Human Mesenchymal Stem Cell Testing
While some manufacturers are taking large-scale adult human Mesenchymal Stem Cell (MSCs) culture into fermentation formats, the majority continue with surface-coated flasks, multi-layered Cell Factories (Nunc) and Cell Stacks (Corning), as well as multilayered coated membrane-based HyperStacks (Corning). Many of these cultured human MSCs are being injected into patients for a variety of health conditions. Accordingly, there is a major need for proper monitoring of human MSCs for clinical trial studies, especially since most are not grown in fermenter culture systems. One of the latter companies, Scharp Technologies (3), has performed preliminary studies using the Cedex Bio Analyzer to document and maintain controlled culture conditions for the cultured adult human MSCs.
While routine monitoring using the Cedex Bio Analyzer can be critical to standard production and GMP manufacturing controls, a recent study shows more interesting use of the Cedex Bio Analyzer in the development of a testing system for MSCs. In order to establish assays for the potential use of MSC conditioned media in consumer and medical products, two different human dermal fibroblast cell lines were obtained from the American Type Culture Collection (ATCC) that each have established optimal culture media for each cell type. For the purpose of this study, these different dermal fibroblast cells, one slow growing (CCL-110) and one fast growing (SCRC-1041), were cultured in the presence of several different culture media: a] fetal bovine serum-supplemented, optimized ATCC medium, b] a basic culture medium, c] Scharp Technologies production medium, and d] Scharp Technologies MSC conditioned medium. In addition, two negative-control media using human AB serum were tested. While the primary study endpoints included a number of parameters such as growth rates and flask surface cell confluence rates, the Cedex Bio Analyzer results provided meaningful information, including sodium and potassium for slow- and fast-growing dermal cells. The differences in sodium and potassium levels in the test media utilized show some minor differences, but no significant or critical results, which is important for GMP production. (See full study for graphical representation of data.)
The glucose and glutamine results are shown in Figure 2A. Examining the glucose results for CCL-110 slow-growing dermal cells, there is a major and unexpected result with the ST7-ATCC Control Medium starting at 5.5 µM glucose when culturing these cells in the different test media. This specific example started with a cell concentration at 40% when plating these cells in T flasks. Looking at the ST7-ATCC Control Medium, these cells dropped their media glucose concentration from 5.5 µM on Day 0 to 3.5 µM on Day 1, with a 50% medium change on Day 3 to 0.8 µM on Day 4, and to essentially 0 µM glucose on Day 7. Both the ST1-Production Medium and the ST3-MSC Conditioned Medium showed similar drops to no measurable glucose remaining in these media by Day 7. The ST5-Minimal Medium had a lesser drop in glucose, while the two Negative Control Media had very small drops in glucose since there was essentially no cell growth in these media. The rapid-growing dermal fibroblasts, SCRC-1041 cells, had their standard ATCC medium starting at a concentration of 24 µM glucose, which dropped during the 7 days of culture with growing cells. The ST5-Minimal Medium and the two Control Media had essentially no drop in glucose concentration for the seven days. However, the ST3-MSC Conditioned Medium still showed a significant drop in glucose levels with seven days of culture, followed by a lesser drop in glucose levels in the ST1-Production Medium. When we changed the starting cell confluence level to 1%, these large drops in glucose concentration did not develop, even when they gradually reached the 40% confluence level. Measuring the glucose levels in these dermal cells growing at different rates is essential information for planning and executing meaningful studies for optimal MSC culture conditions.
Examination of the glutamine levels in Figure 2A in these same media with the different dermal fibroblasts shows that the ATCC control media for the slow-growing cells (CCL-110) and for the rapidly growing cells (SCRC-1041) both reduce their glutamine levels significantly over the 7 days. The ST5-Minimal Medium as well as the ST2-Negative Control Medium had very low glutamine levels to start. The ST1-Production Medium, the ST3-MSC Conditioned Medium, and ST4-Negative Control Medium all had higher levels of glutamine at the start that varied in levels over the seven days of culture monitoring.
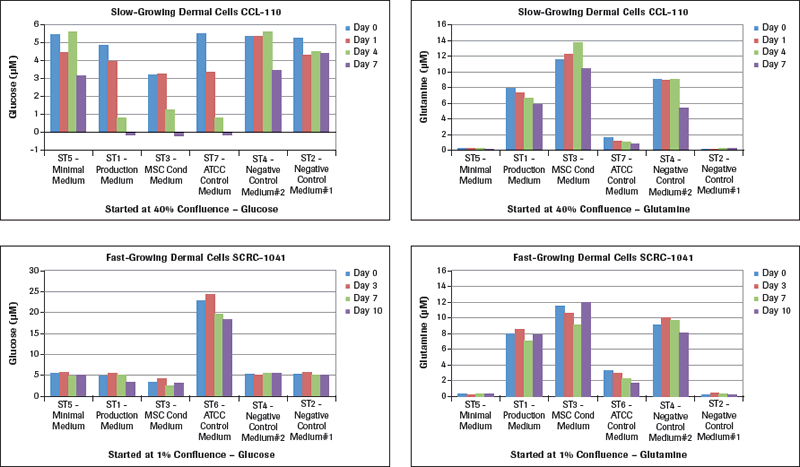
Lactate measurements in culture media provide a representation of the degree of metabolism the cultured cells are capable of providing. While we learned how much glucose was utilized (Figure 2A) by these two different types of dermal fibroblasts cultured in different media, we now see in Figure 2B that the maximal lactate levels representing cell metabolism were achieved in the slow-growing CCL-110 cells which also had dropped their glucose concentration nearly to zero levels. The ST5-Minimal Medium had significantly lower lactate production also coordinating with their glucose levels. For the rapidly growing SCRC-1041 dermal cells, the most lactate production was observed using the ST6-ATCC Control Medium, with lesser amounts produced in the ST1 Production Medium and the ST3-MSC Conditioned Medium.
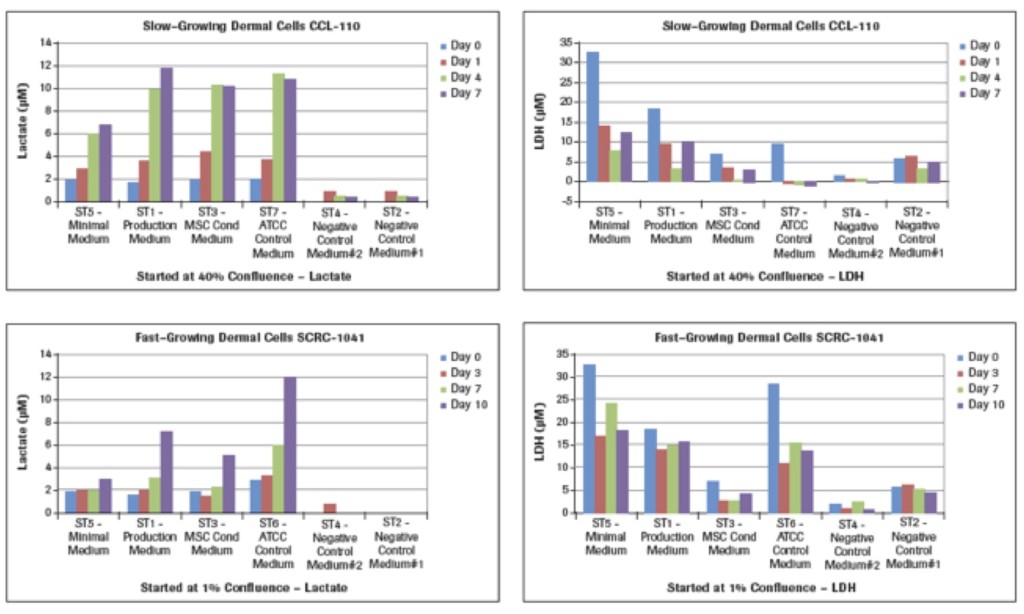
The LDH results in Figure 2B represent cell death in these cultures. Analysis of the LDH levels shows that the largest concentrations at the different times for both cell types are from the use of the ST5-Minimal Medium at all of the times. The lowest LDH levels are recorded with use of the ST3-MSC Conditioned Medium, with the ST1-Production Medium being intermediate. Of interest are the results for the ATCC control media in that the ST7-ATCC Medium for the slow-growing CCL-110 dermal cells resulted in very low levels of LDH. However, the much higher glucose-containing ST6-ATCC Medium for the rapid-growing SCRC-1041 dermal cells also had much higher LDH levels, suggesting higher death of those cells.
Ammonia levels were also tested for these cell cultures. (See full study for graphical representation of data.) The ammonia levels are in part timed to the glutathione/glutamine levels that when metabolized produce ammonia. The Control Medium #1 and the Minimal Medium are essentially the same medium with low levels of glutamine and glutathione. The only difference between these two media is that the Control Medium #1 is supplemented with human AB serum, while the Minimal Medium is supplemented with fetal calf serum (FBS). The ammonia levels in both of these media are very low since the nutrients for producing ammonia are low.
In contrast, the Control Medium #2 has high levels of glutamine and glutathione and without supplementation is the same as the Production Medium. The Control Medium #2 also is supplemented with human AB serum, while the Production Medium is supplemented with FBS. The Conditioned Medium is the same as the Production Medium except that it has previously cultured human MSCs and, as shown, has a little lower glutathione and glutamine due to its exposure to cell culture. The two ATCC media have small differences in glutathione and glutamine levels, explaining their differences in ammonia levels. Thus, the use of the Cedex Bio Analyzer clearly shows by this example its importance in its ability to measure these types of nutrients and metabolites as a major control in GMP MSC cell production.
Conclusion
Use of the Cedex Bio Analyzer for testing culture media from growing cells of different types provides essential information regarding the viability and function of the cultured cells under different conditions. This study suggests that the Cedex Bio Analyzer can be critical in monitoring routine primary cell cultures into long-term culture under standard production and GMP manufacturing controls. It can also be utilized in maintaining controlled culture conditions for tissue culture by measuring key metabolic substrates such as glucose, lactate, LDH, and glutamine during cell growth. Furthermore, analysis using the Cedex Bio Analyzer can provide critical information in monitoring and developing MSCs, from culture medium development and monitoring through GMP processing.
Click here to access the full study.
Click here to learn more about the Cedex Bio Analyzer.
The Cedex Bio is for use in quality control/manufacturing processes only.
References
- Scharp D, Arulmoli G, Taeidi P, Albert E, Avakian A, Sunshine H, Kucera C, McRaney D, Bondurant D, Haight B, Magaldi M. Survival and function of human islets cultured for 4 weeks post-processing. IPITA 2013 Abstracts. September 27, 2013.
- Scharp D, Arulmoli G, Albert E, Kucera C, Sunshine H, Avakian A, Taeidi P, McRaney D, Bondurant D, Haight B, Magaldi M. Multi-analysis of islet culture variables for human islet culture optimization. IPITA 2013 Abstracts. September 27, 2013.
- Scharp Technologies, Inc. home page. http://www.scharptechnologies.com.