
Minimize the Impact of Stress on Pluripotent Stem Cell Health in a Range of Workflows
Pluripotent stem cells (PSCs) are foundational tools for basic research, as well as applied applications including regenerative medicine, Gene Therapy, drug discovery, and toxicological assessment. While stem cells have been shown to have a tremendous proliferative capacity, stress on the cells from long term culture has been shown to cause an accumulation of mutations that result in genetic instability, increasing the tumorigenicity of the cells and thus limiting their utility in research and clinical applications. Within the process of standard culture, PSCs undergo significant cellular stress during cryopreservation, post-thaw recovery, as well as passaging. Downstream processes, such as gene editing, apply additional cellular stress, comprising cell viability and furthermore genetic stability of PSCs. Thus, solutions to minimize the impact of cellular stress during these workflows are essential components to ensure reliability and consistency of cell supply, as well as experimental reproducibility.
Mechanisms of Cryopreservation-Induced Cellular Injury
Cryopreservation solutions provide experimental flexibility and consistency of cell supply, allowing for generation of banks of early passage PSCs. However, cellular damage as a result of the cryopreservation process can significantly impact post-thaw recovery and in some instances result in loss of cell lines. During the process of cryopreservation, cellular damage occurs via intracellular and extracellular ice formation, dehydration, and increasing concentration of solutes within the cell. To assist in mitigation of this cellular stress, slow-rate cooling methods in which cells are cooled to -80 °C at a rate of ~-1 °C/minute and subsequently transferred to an LN2 dewar are implemented. These slow-rate cooling methods allow for gradual efflux of water from the cytosol of the cell, reducing the potential for intracellular ice formation and slowly changing the concentration of solutes within the cell. Additionally, inclusion of cryoprotectants such as DMSO, propylene glycol, or ethylene glycol are utilized to lower the freezing temperature, thus reducing formation of intracellular and extracellular ice. Cryoprotectants additionally permeate the cells to allow for replacement of water within the cells, assisting in minimizing the impact of cell shrinkage and fluctuation in the concentration of solutes within the cell.
While cryoprotectants are utilized to assist in minimizing the cellular stress during the cryopreservation process, they are also toxic to the cells. This toxicity is time and temperature dependent, with higher concentrations and higher temperatures resulting in the greatest toxic penalty on the cells. Additionally, during the removal of cryoprotectants from the cell, abrupt changes in the cell volume results in membrane rupture via osmotic shock, thus immediately compromising the viability of the cells. Due to this balance of protection and toxicity, while the direct post-thaw viability of PSCs cryopreserved in various solutions is often times high, over the first 24 hours significant apoptosis and necrosis occurs due to the additive effects of the cellular stress outlined above. Therefore, solutions and procedures are incredibly important to achieve optimum post-thaw recovery of PSCs.
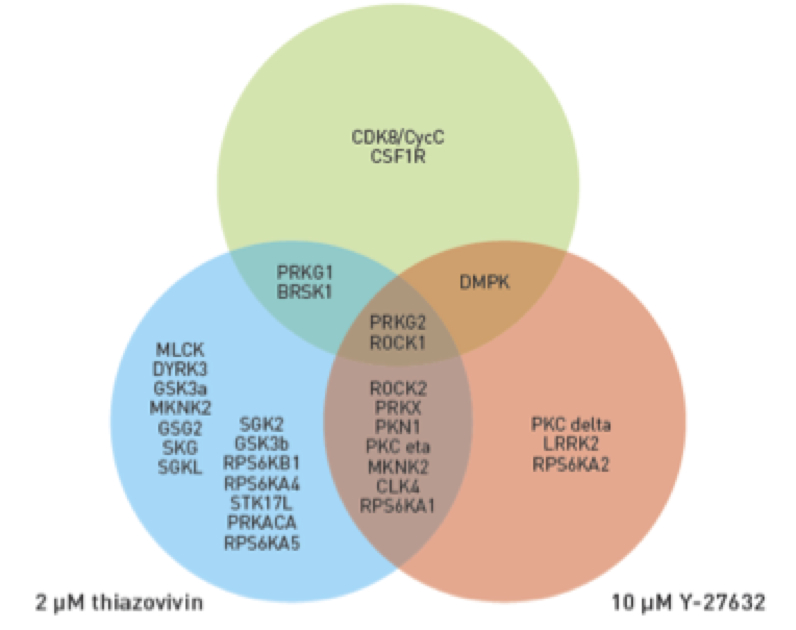
To mitigate the impact of cellular stress during recovery of PSCs, small molecules such as Y-27632 or thiazovivin have traditionally been added to the culture medium for the first 24 hours post-thaw to inhibit the rho kinase associated coiled-coil proteins, thus inhibiting actin cytoskeletal rearrangement, and ultimately preventing apoptosis of PSCs. However, in addition to inhibition of ROCK activation, Y-27632 and thiazovivin are shown to inhibit a number of other protein kinases, impacting a number of off-target pathways (Figure 1). In addition, these small molecules do not address the formation of reactive oxygen species which become plentiful in the cells over the course of the cryopreservation and recovery processes. Improved solutions are required to optimize cell viability over the first 24 hours post-thaw, as well as provide more selective small molecules to minimize the impact of potential off-target effects.
Figure 1:
Introducing Thermo Fisher Scientific’s PSC Cryopreservation Kit-Efficiently Reducing the Impact of Stress During the Cryopreservation Process
Thermo Fisher Scientific’s PSC Cryopreservation Kit (Cat #A26446-01) contains a ready-to-use, xeno-free PSC Cryomedium coupled with an animal-origin-free, chemically-defined recovery supplement−RevitaCell™ Supplement (Cat #A26445-01). When used together, these solutions provide optimum cryopreservation and recovery of PSCs, minimizing loss of cell viability and thus maximizing post-thaw recovery. Some of the key features of this system include:
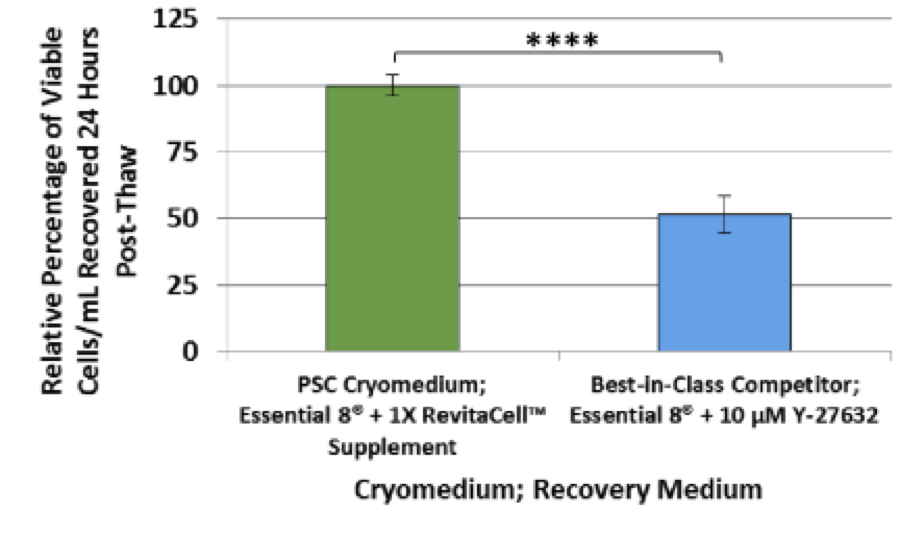
- Efficient recovery – Routinely achieves 50% more viable cells 24 hours post-thaw than when using best-in-class competitive cryopreservation solutions followed by recovery in growth medium supplemented with Y-27632 (Figure 2).
Figure 2
- Consistent – Optimized solutions and cGMP manufacturing processes provide optimum quality of cryopreservation and recovery solutions.
- Easy to Use – The PSC Cryopreservation Kit is provided as ready-to-use components, saving valuable time.
- Versatile – The PSC Cryopreservation Kit is compatible with clump and single cell passaged PSCs cultured in a variety of media systems, including Essential 8®, StemPro hESC SFM, and other commercially available formulations.
- RevitaCell™ Supplement contains a more specific ROCK inhibitor coupled with antioxidants and free radical scavengers which inhibits formation and protects cells against ROS build-up.
- Additionally can be used in cryopreservation and recovery of human peripheral blood mononuclear cells, providing a xeno-free alternative for cryopreservation.
Additional Utility of RevitaCell™ Supplement to Support Cells During Stress Associated with Single Cell Passaging
Although the advent of feeder-free culture systems greatly improved the scalability of PSCs, the use of clump passaging techniques (e.g., using reagents such as dispase, collagenase, or EDTA) has often been incompatible with downstream experimental applications, such as high-throughput screening, gene editing, and directed differentiation.
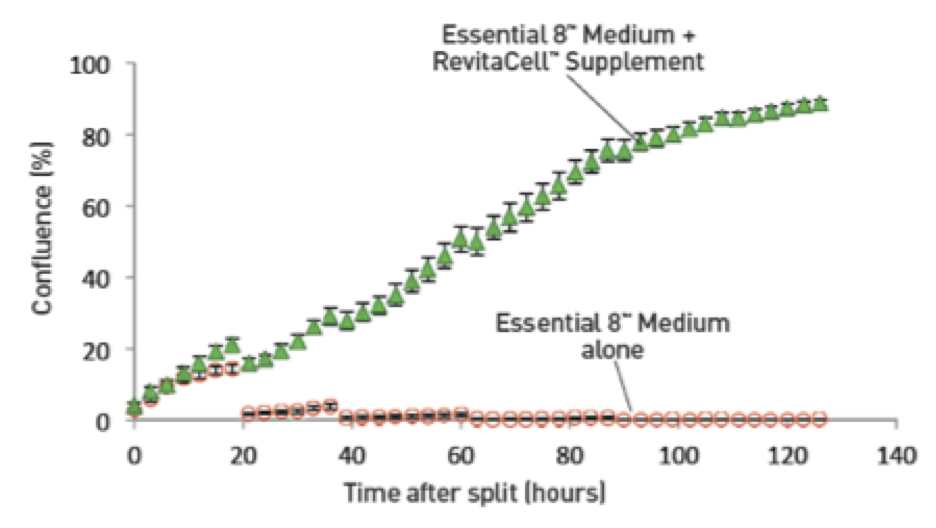
Single cell culture is a standardized and accepted practice in some areas of stem cell culture research. By employing techniques passage using single cell dissociation, scientists are able to have dependable cells for downstream applications and a basis of comparison for clone selection, imaging, cell sorting, etc. where cell recovery and cell number are vital to success. The RevitaCell™ Supplement has shown additional utility in the context of single cell passaging using Essential 8™ medium (Figure 3).
Figure 3
For additional information, assistance in selecting the right cryomedium for your research, and application notes on these products please visit the following links:
- www.lifetechnologies.com/essential8scp & www.lifetechnologies.com/cryopreservation.
- Cell Culture Dish – Ask the Expert Session – Minimize the Impact of Stress on Pluripotent Stem Cell Health – A Discussion