Optimization of Roche Liberase MNP-S GMP Grade in the Enzymatic Digestion of Human Umbilical Cord for the Isolation of Mesenchymal Stem Cells
A Guest Blog by Colleen Cloud¹, Norma Graber¹, Tara Duke¹, Jingjing Wang¹, Hongjun Wang¹ and Gary Gilkeson²
1 Department of Surgery, Center for Cellular Therapy
2 Division of Rheumatology and Immunology, Medical University of South Carolina Charleston, SC 29425
Introduction
Mesenchymal stem cells were originally developed for medical purposes to improve wound healing. They were then found to have significant immune effects, primarily immune suppressant and tolerance inducing effects. There are successful reports of using MSCs to treat animal models of a number of autoimmune diseases. Mesenchymal stem cells can be derived from many different sites, including bone marrow, dental pulp, adipose tissue and umbilical cords. In our studies of murine models of lupus, umbilical cord-derived MSCs had the best therapeutic profile compared to bone marrow derived MSCs from controls.
To translate this to a clinical level, the goal was to find a GMP-grade enzyme that would safely and efficiently digest human umbilical cord tissue to derive mesenchymal stem cells for further use. Previous qualifications were performed using a non-GMP grade enzyme for the purpose of establishing a protocol. Once the feasibility of deriving MSCs from umbilical cord tissue was determined, the challenge was to implement the use of a GMP-grade enzyme. Here we describe the results from four qualifications, indicating the Liberase MNP-S is an ideal candidate for isolating mesenchymal stem cells from umbilical cord tissue.
Materials and Methods
Consent was obtained from healthy mothers undergoing a live birth C-section for the umbilical cord procurement. Fresh cords were transported at room temperature to the laboratory for immediate processing. After measuring the cord length, vasculature was removed from the cord and any remaining blood was rinsed from the tissue in DPBS. The tissue was then minced into pieces measuring 3 mm x 3 mm or smaller in a petri dish containing alpha-MEM culture medium. For every 5 cm of cord, the resulting volume of minced tissue and α-MEM was measured to be 10 mL. The 10 mL of minced tissue/α-MEM was transferred to a 50 mL conical tube and placed on ice during enzyme preparation. Liberase MNP-S (5 mg) was prepared at various concentrations by dissolving the lyophilized disc with α-MEM. An equal amount of enzyme was added to the conical tube containing the minced tissue. The addition of Hylenex® (Halozyme Therapeutics) was titrated based on the digestion times. After digestion, the tissue pellet was washed several times before resuspension in complete culture medium. Cells were cultured in a 37°C incubator with 5% CO2 and passaged when cells reached 80-90% confluence. Based on our needs for a clinical approach, the MSCs were cryopreserved at passage 2. Flow cytometry was performed on P2 cells prior to freezing for phenotypic analysis.
Results
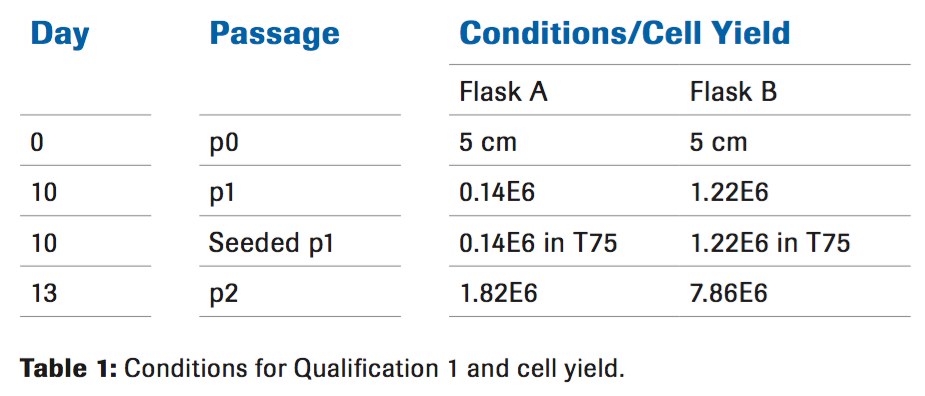
Qualification 1
Liberase MNP-S was diluted to a final concentration of 50 ug/ ml. Due to limitations of the initial cord length, two conditions were performed (with or without Hylenex®). The tissue was placed on a rocker in a 37°C incubator for 30 minutes. After the 30 minute incubation, the enzyme reaction was stopped by performing multiple washes with ice cold PBS. At this point the tissue appeared to be approximately 30 percent digested, meaning pieces of solid tissue were visible. The cells were resuspended in complete culture medium and seeded in a tissue culture flask. A medium exchange was performed after 72 hours allowing time for the cells to adhere to the flask. Upon removal of the supernatant it was noted that the addition of Hylenex® eliminated the viscosity that was present in the absence of Hylenex®. At passage 2, when the cells were being prepared for cryopreservation, a small aliquot was used for counting by Trypan blue in addition to flow cytometric analysis.
The cell yield indicates that the use of Hylenex in combination with the Liberase MNP-S is needed to obtain a higher number of cells (Table 1). Since the cell yield was significantly lower than previous qualifications using the non-GMP enzyme from an alternate manufacturer, further qualifications were needed to improve the cell yield. Based on pre-qualification data, using a higher concentration of Liberase MNP-S for a longer digestion time resulted in over digestion of the tissue. For future qualifications it was decided to titrate the amount of Hylenex® in addition to decreasing the Liberase concentration and increasing the digestion time.
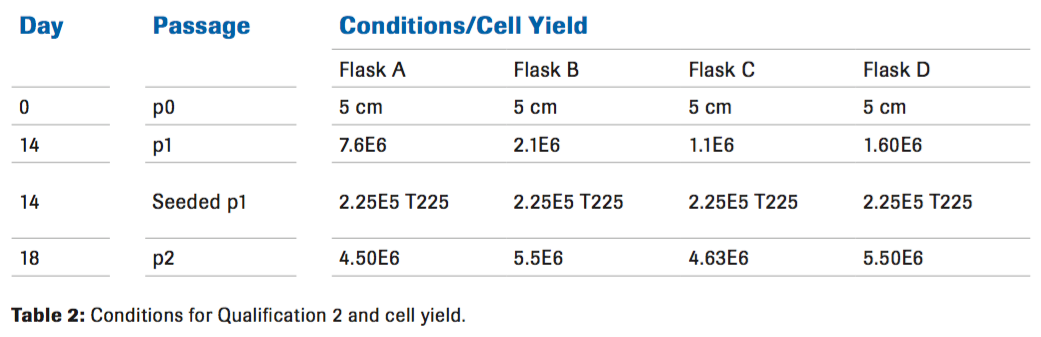
Qualification 2
A fresh cord from a different donor was used to perform a second qualification. The cord was processed and digested as previously described for 30 minutes with 50 ug/mL of Liberase while varying the amount of Hylenex (Table 2). There was no difference in cell yield at passage 2 with the higher concentrations of Hylenex. Cell yield also decreased. Based on this information, a third qualification was needed.
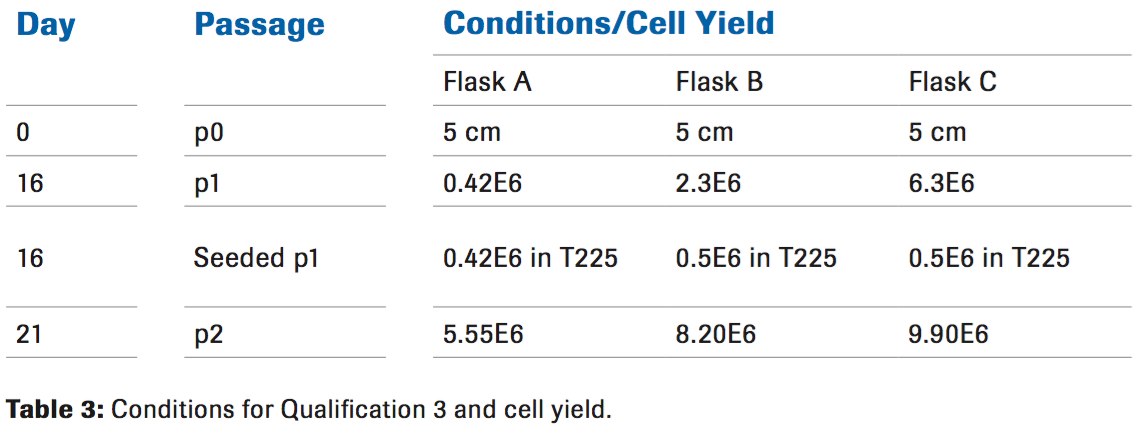
Qualification 3
Going in a different direction from qualification 2, a lower dose of both Liberase and Hylenex were used while increasing the digestion time (Table 3). The increased cell yield was a promising indication that we were closer to the optimal conditions. Increasing the digestion time to 60 minutes and decreasing the Liberase to 25 ug/mL allowed for more cells to be released from the tissue as seen at passage 1.
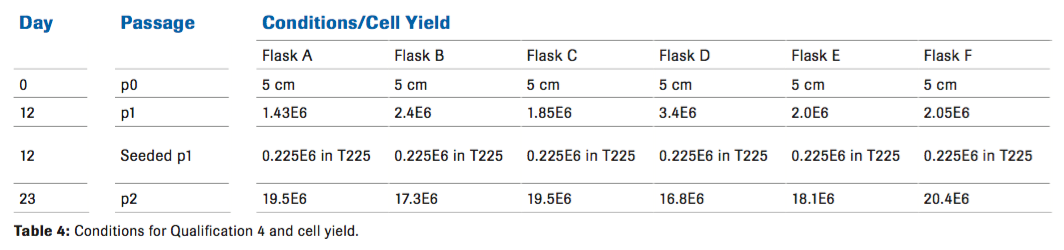
Qualification 4
A final qualification was performed to see if the results from qualification 3 could be repeated, while further titrating the amount of Hylenex, in order to increase the cell yield without reintroducing a viscous environment to the cells. Cell growth was not affected at the end of passage 2 which was expected, however, we did see a difference in cell yield at passage 1 with an increased number of cells compared to the other conditions (Table 4). Although the length of time that it takes to reach passage 2 was variable, we did not see any phenotypic differences.
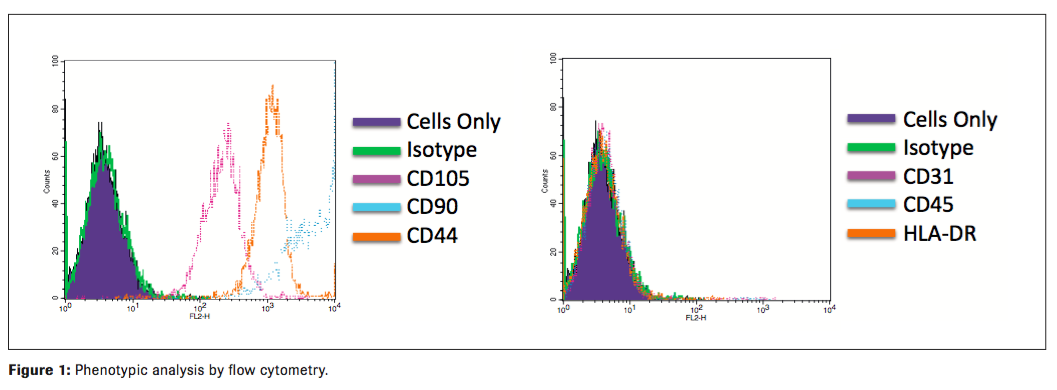
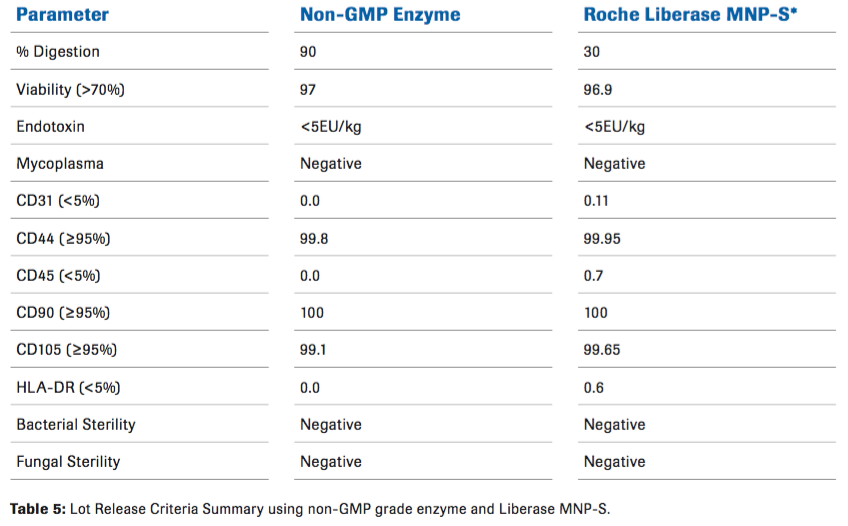
The MSCs from all qualifications were found to possess the expected immunophenotype of being CD44, CD105, and CD90 positive and negative for CD45, CD31, and HLADR. Contamination by hematopoietic stem cells (CD34+, CD45+), B-cells (CD19, CD45+), monocytes/macrophage (CD11b, CD45+) and endothelial (CD34+, CD45) cells were cumulatively all less than 5 % (Figure 1). They are free of bacteria, mycoplasma, and endotoxin. They possess strong immunosuppressive functions as measured by T cell suppressive assay (data not shown). All qualifications met Lot Release criteria for future clinical applications. (Table 5)
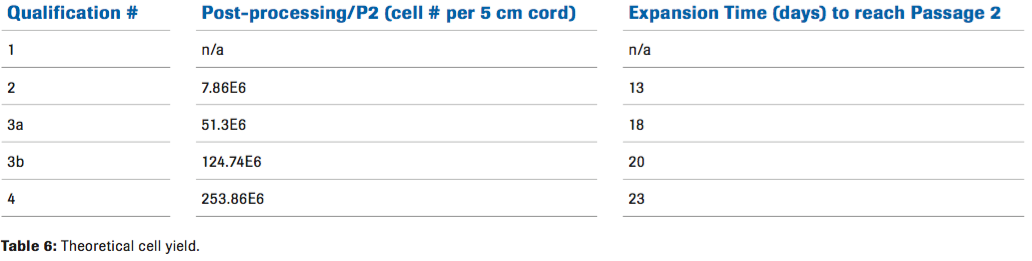
For potential clinical applications, being able to produce enough MSCs was critical. Based on the results from qualification 4, we were able to calculate a theoretical cell yield. Using the optimal condition from this qualification, we calculated that approximately 253 million cells can be generated from one 5cm cord. (Table 6).
Conclusion
Liberase MNP-S can be used effectively to generate MSCs from human umbilical cords. After these qualifications, we found that Liberase MNP-S can be used at 50 ug/mL or 25 ug/mL depending on the digestion time. Due to limitations of donor availability, further analysis is needed to address the percentage of tissue that was digested. Liberase MNP-S can be used to successfully generate MSCs for large scale production.
For more information on Liberase Enzyme Blends, visit www.custombiotech.roche.
Regulatory disclaimer – For further processing only.
Footnotes
-
1. Augello, A., Tasso, R., Negrini, S. M., Cancedda, R. & Pennesi, G. Cell Therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 56, 1175–1186 (2007).
-
2. González, M. A., Gonzalez-Rey, E., Rico, L., Büscher, D. & Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60, 1006–1019 (2009).
-
3. Chang, J.-W. et al. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant 20, 245–257 (2011).
-
4. Sun, L. et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum 62, 2467–2475 (2010).