3D Bioreactor Platform Provides Increased Process Control and Throughput for Spheroid and Organoid Generation and Culture
Conventional adherent two-dimensional (2D) tissue culture where cells are grown on planar, rigid substrates has been the basis for many scientific discoveries. However, as cell culture techniques have advanced to grow cells in three-dimensional (3D) space, comparative studies with 2D cultures demonstrate they are simply unable to recapitulate the complex tissue architecture and cell connectivity observed in vivo. This can limit their utility to model cellular morphology, viability, proliferation, differentiation, gene and protein expression, and responses to drug and toxic compounds. Because of this, biomimetic 3D cell culture models, such as spheroid and organoids have gained broader acceptance for translational research, drug discovery and toxicology programs.
The use of human cell-based 3D systems drastically improves the predictive power of in vitro testing to bridge the gap between 2D cell culture and native tissues, which also aids in the replacement of animal models for toxicity and drug testing which suffer from high cost, ethical issues, and lack of predictivity of the human response. The high attrition rates for drug candidates stemming from unidentified adverse or toxic side effects during early preclinical studies using 2D cell-based assays and animal testing highlights an urgent need for better technologies to improve precision in drug discovery. 3D cell cultures more accurately reflect human tissues (morphology, polarity, cell–cell communication, microenvironmental cues, proliferation rates, and gene and protein expression) making them better suited for the evaluation of cellular responses to various compounds or drugs.
However, 3D cultures are not without their challenges. Scaffold-free culture is often preferred for drug and toxicity programs because some scaffolds can impart biological activity that can confound results, but scaffold-free cultures often suffer from heterogeneity in shape and size, and poor viability which can impact their natural response to treatment. Also, it takes time for cells to reach metabolic equilibrium, self-organize, grow and mature into 3D structures that function like tissue. Thus, there is a need for technologies to increase the throughput of 3D cultures, as well as enable their extended culture periods to establish in vivo-like tissues to recapitulate physiological responses.
CelVivo ClinoStar System
The ClinoStar system by CelVivo is a 3D bioreactor platform developed to address the technology gap for 3D cultures. The technology is based on the clinostat principle also referred to as a simulated microgravity system. A clinostat, (ClinoStar), and a rotating bioreactor (ClinoReactor) provides a scaffold-free culture environment that keep the cells suspended by exerting gravitation forces on the cells from all sides. This enables the generation of large numbers of uniform spheroids or organoids using very low shear forces while facilitating gas and nutrient exchange. The system is equipped with cameras for ease of real-time monitoring throughout the culture period. Combined with tablet-enabled control of culture parameters like temperature, CO2 and rotational speed enables enhanced process control. In addition, the closed system design mitigates contamination risk.
To highlight some of the diverse applications in which the CelVivo ClinoStar bioreactor platform has been used, we summarize some recent publications here.
1) METABOLIC RECOVERY OF HEPATOCYTES AFTER TRYPSINIZATION IN 2D AND 3D CULTURE
Trypsinization is a technique used widely in the culture of adherent cells in 2D. Despite this and the knowledge that trypsinization sends cells into a state of wound healing that alters gene expression and suppresses physiologically important functions, little work has been done to determine when the cells are fully recovered from the damage caused by this stress. In two publications1,2, the cellular recovery from regular trypsinization on both 2D monolayers and 3D spheroids formed in the CelVivo bioreactor with HepG2-C3A hepatocytes was examined.
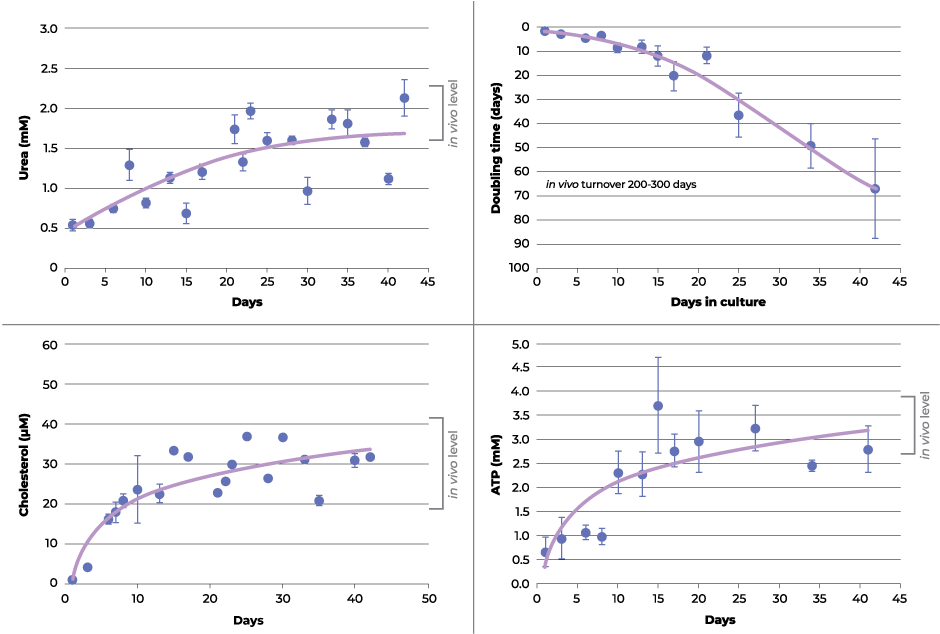
The authors found that the recovery rates post-trypsinization in both 2D monolayers and 3D hepatocyte spheroids was similar. However, the 2D monolayer cultures reach confluence and require subculturing after 5 days, which resets the wound healing phase while 3D spheroids can be maintained in culture for much longer extending the recovery period. It is after ~18 days when changes in growth rate, extracellular adenylate kinase, adenosine triphosphate (ATP), urea and cholesterol are observed in the 3D spheroids suggesting a physiological transition back to metabolic equilibrium is occurring (see figure 1). Similar reports with other cell lines in 3D culture indicate these changes may be a ubiquitous recovery process taking 2-3 weeks after trypsinization before they begin to mimic in vivo cytoarchitecture. The extended culture period when cells are grown in 3D allows them to recover and exhibit biomimetic features that simply isn’t possible within the constraints of 2D monolayer culture due to repetitive wound healing cycle.
2) LIVER-MIMETIC SPHEROIDS TO MODEL ACUTE AND CHRONIC DRUG TOXICITIES
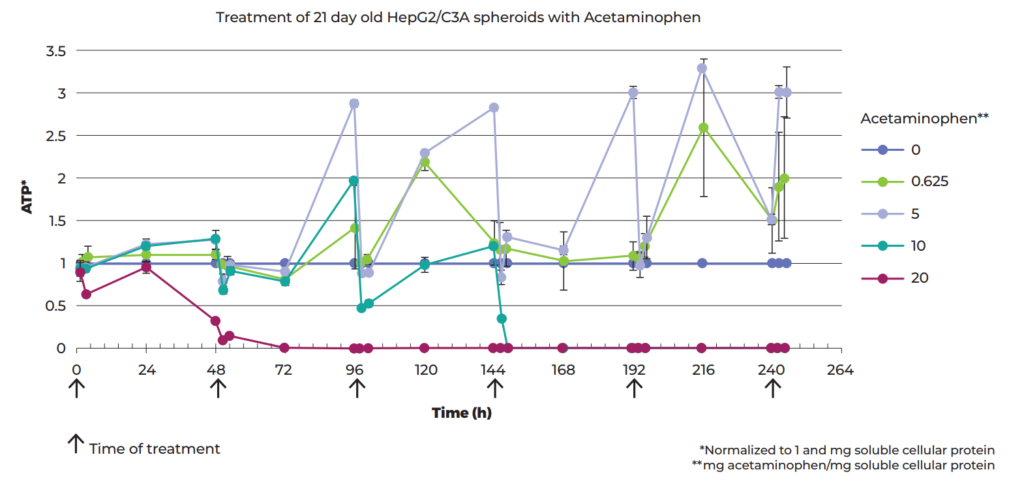
Prescription medications are often prescribed to patients as repeat doses for a varied length of time, however, animal toxicity studies with repetitive drug treatments show poor correlation with human clinical observations. Therefore, better in vitro human cell-based models are needed to assess repeated-dose drug toxicity. One potential approach uses CelVivo clinostat-cultured 3D HepG2–C3A liver-mimetic spheroids. In this study3,4, both acute and chronic repeated-dose studies of six drugs (amiodarone, diclofenac, metformin, phenformin, acetaminophen (APAP or paracetamol) and valproic acid) were evaluated in the hepatocyte spheroid model to assess its predictivity.
First, the LD50 (assessed by ATP production) of each of the six drug candidates was determined by treating 21-day-old C3A spheroids with doses varying by orders of magnitude. Then, to look at accumulated toxicity from repeated-dosing, spheroids were treated six times with various doses of acetaminophen (commonly known as paracetamol) at 48-h intervals during a 10-day period where the response to all doses became stronger after the first two treatments (see Figure 2). The therapeutic index between the single and repeated-dose thresholds for lethal toxicity was calculated to be 3-fold lower than commonly assumed, which could be of significant clinical relevance.
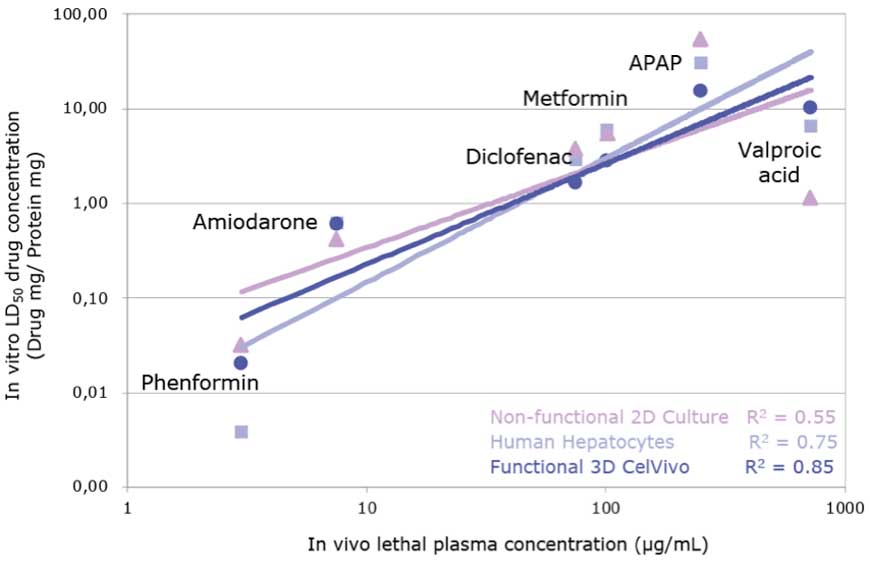
Additionally, the correlation for six drugs between the LD50 in HepG2-3A spheroids against the lethal dose in vivo was better than the correlation with 2D-cultured HepG2 cells and primary human hepatocytes suggesting that spheroids are more predictive of human in vivo toxicity (see Figure 3). These results led the authors to conclude that the HepG2-C3A spheroids could be a practical model suitable for the determination of the repeated-dose LD50 over the course of several days. In combination with the CelVivo system to generate robust and reproducible spheroid cultures, it is possible to test repeated drug treatments over extended periods of time in a consistent manner.
3) IN VITRO MODEL OF BONE STRENGTH
3D bone spheroids, also called osteospheres, are a novel in vitro model that can be leveraged to study the molecular mechanisms of bone remodeling and the pathophysiology of bone diseases. Previous research has shown the utility of scaffold-free osteospheres formed from primary human osteoblasts in the CelVivo bioreactors to study the biomechanical properties of bone in response to external stimuli.
Lack of dietary vitamin D and K is known to increase bone fracture risk, which suggests that dietary supplementation with these vitamins could be beneficial to increase bone strength. Combined administration of vitamin D and K is suggested to have synergistic positive effects on calcium homeostasis and bone health. In this publication highlight5, the authors looked to identify the effect of these vitamins on the gene expression and secretion of proteins and cytokines involved in the biological and mechanical functions of bone in both 2D cell cultures of primary osteoblasts and in 3D osteospheres.
With the two vitamins in combination, enhanced gene expression of periostin and COL‐1, important for bone stiffness, was observed along with increased osteoid formation and mineral deposition, hallmarks of bone strength. The testing revealed the two vitamins have differential effects on bone mechanical properties where vitamin D enhancing stiffness while vitamin K imparts flexibility to bone but overall, the combined treatment has the potential to increase fracture resistance in vivo. In the testing, the authors found the 3D cultures were better able to mimic in vivo tissue functionality than the 2D monolayers making them more suited to evaluate cellular responses to various compounds or drugs.
4) IN VITRO COLORECTAL CANCER SPHEROID MODEL TO SCREEN CANCER THERAPEUTICS
Colorectal cancer is the third most commonly occurring cancer in men and the second most commonly occurring cancer in women illustrating the desperate need for effective drug treatments. However, the success rate of new candidate cancer drugs in clinical trials remains dismal due in part to the inability of traditional 2D cell culture-based screening platforms to accurately predict in vivo physiological responses. In this study6, a colorectal cancer 3D spheroid system was developed to evaluate its efficacy in screening anti-cancer treatments.
LS180 colorectal cancer cells were cultured as 3D sodium alginate encapsulated spheroids in the CelVivo clinostat bioreactors. To establish baseline responses for this model, the colorectal spheroids were treated with a standard chemotherapy drug, paclitaxel and IC50 (half maximal inhibitory concentrations) was determined using the 3- (4,5- dimethylthiazol- 2- yl)-2,5-diphenyltetrazolium bromide assay. Soluble protein content, intracellular ATP levels, extracellular adenylate kinase, glucose consumption, and P-glycoprotein gene expression were measured. To further validate the model for drug screening, two concentrations of paclitaxel were compared to untreated control cells. Paclitaxel was found to decrease cell growth, viability, and glucose consumption in the model with concomitant increases in expression of P-glycoprotein compared to the control. This overexpression of P-glycoprotein is typical in cancer tissues observed in vivo as a mechanism of drug resistance to paclitaxel treatment. From the data collected, the authors suggest that the LS180 sodium alginate encapsulated spheroid model has the potential to be used for testing new chemotherapeutic compounds for colorectal cancer.
5) COMPARISON OF A SPHEROID AND ANIMAL MODEL TO EVALUATE HEPATOTOXICITY
Xysmalobium undulatum is one of the most widely used traditional medicinal plants in Southern Africa, but there is belief that its use may cause liver damage. In this publication7, the authors looked at the hepatotoxicity (cell viability and proliferation parameters measured) of X. undulatum extract in comparison to a known hepatotoxin in a sub-chronic study (21 days) with both the in vitro 3D HepG2/C3A spheroid model (see Figure 4, images courtesy of CelVivo) and the in vivo Sprague Dawley rat model.
HepG2/C3A spheroids were cultured in the CelVivo system and treated with a known hepatotoxin, valproic acid, and crude X. undulatum extract for 21 days. Spheroid growth, intracellular ATP levels and extracellular adenylate kinase were used as readouts for hepatotoxicity. The Sprague Dawley rat model was treated similarly over 21 days and changes in aspartate aminotransferase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH) serum levels were used to quantify in vivo toxicity. The results from both the spheroid and animal model confirmed the hepatotoxicity of valproic acid and both models were able to show a potential concentration-dependent hepatotoxicity of the X. undulatum extract. This side-by-side comparative study demonstrates the predictive capabilities of the in vitro 3D HepG2/C3A spheroid model similarly to traditional in vivo animal model systems like the Sprague Dawley rat model. Studies like the one here provides more evidence that 3D spheroid models could eventually replace animal testing to assess drug toxicities.
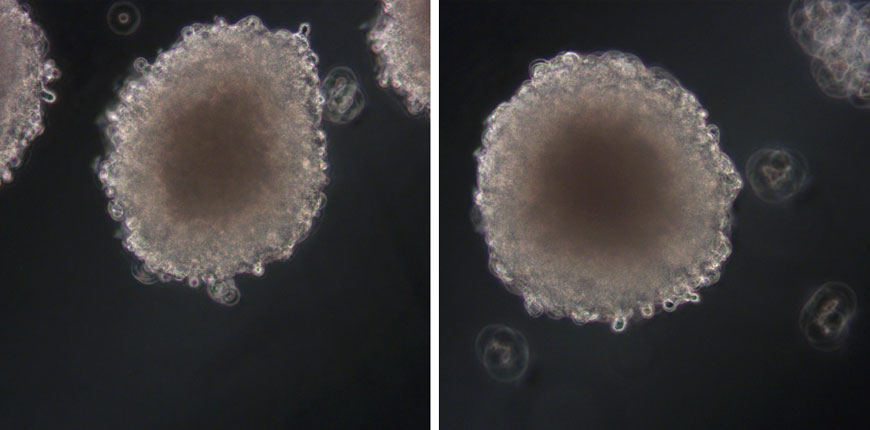
Figure 4
Footnotes
-
1. Wrzesinski K. and Stephen J. Fey SJ. After trypsinisation, 3D spheroids of C3A hepatocytes need 18 days to re-establish similar levels of key physiological functions to those seen in the liver. Toxicol. Res. 2013;(2): 123-135. Published 2012 Dec 4. doi: 10.1039/C2TX20060K
-
2. Krzysztof Wrzesinski, Maria Chiara Magnone, Line Visby Hansen, Marianne Ehrhorn Kruse, Tobias Bergauer, Maria Bobadilla, Marcel Gubler, Jacques Mizrahi, Kelan Zhang, Christina M. Andreasen, Kira Eyð Joensen, Signe Marie Andersen, Jacob Bastholm Olesen, Ove B. Schaffalitzky de Muckadell, Stephen J. Fey, HepG2/C3A 3D spheroids exhibit stable physiological functionality for at least 24 days after recovering from trypsinisation, Toxicology Research, Volume 2, Issue 3, May 2013, Pages 163–172,
-
3. Fey SJ, Korzeniowska B, Wrzesinski K. Response to and recovery from treatment in human liver-mimetic clinostat spheroids: a model for assessing repeated-dose drug toxicity. Toxicol Res (Camb). 2020;9(4):379-389. Published 2020 Jun 12. doi:10.1093/toxres/tfaa033
-
4. Wojdyla K., Wrzesinski K., Williamson J., Fey SJ., Rogowska-Wrzesinska A. Acetaminophen-induced S-nitrosylation and S-sulfenylation signalling in 3D cultured hepatocarcinoma cell spheroids. Toxicol Res (Camb). 2016 May 1; 5(3): 905–920. Published online 2016 Mar 2. doi: 10.1039/c5tx00469a
-
5. Schröder M, Riksen EA, He J, et al. Vitamin K2 Modulates Vitamin D-Induced Mechanical Properties of Human 3D Bone Spheroids In Vitro. JBMR Plus. 2020;4(9):e10394. Published 2020 Aug 3. doi:10.1002/jbm4.10394
-
6. Smit T, Calitz C, Willers C, et al. Characterization of an Alginate Encapsulated LS180 Spheroid Model for Anti-colorectal Cancer Compound Screening. ACS Med Chem Lett. 2020;11(5):1014-1021. Published 2020 Apr 3. doi:10.1021/acsmedchemlett.0c00076
-
7. Calitz C, Hamman JH, Fey SJ, Viljoen AM, Gouws C, Wrzesinski K. A sub-chronic Xysmalobium undulatum hepatotoxicity investigation in HepG2/C3A spheroid cultures compared to an in vivo model. J Ethnopharmacol. 2019;239:111897. doi:10.1016/j.jep.2019.111897