A High Throughput System for CAR-T Cell Screening in Tumor Spheroids
Introduction
Chimeric antigen receptor (CAR)-T cells which are engineered to recognize tumor cell-specific surface antigens, have shown promise to affect complete remission in patients with B-cell malignancies. With the recent FDA approval of Novartis’ Kyrmriah™ and Gilead Science’s Yescarta™, CAR-T Cell Therapy could be on target to become the standard treatment for blood cancers1. However, utilizing the CAR approach to treat solid tumors has not been as successful because many of the surface antigens upregulated in solid tumor cells, and thus chosen as targets for CAR-T cells, are present at significant levels in normal tissues2. This has resulted in significant adverse effects in clinical studies including toxicity to healthy tissues and at worst, has proven fatal3. Methods for testing different models of CAR-T cells in vitro can provide further insight into viable antigen targets before first-in-man studies that utilize such models. The latest advancements in drug discovery screening, especially for cancer therapeutics, utilize three-dimensional (3D) cell culture models which more closely mimic the in vivo tumor microenvironment. For example, 3D multicellular tumor spheroids develop hypoxic cores, demonstrate gradients of various soluble factors, and a diffusion profile for drugs similar to tumors4. Here we demonstrate an HTS-compatible system to assay CAR-T cells against tumor spheroids by combining Corning® spheroid 384-well ultra-low attachment microplates, which enables the reproducible formation of a single spheroid in each well, and the DiscoverX KILR® Cytotoxicity assay, which provides a non-radioactive, dye-free method to specifically measure target cell death in a co-culture using luminescence.
Materials/Methods
KILR Retroparticles Transduction
In this study, two tumor cell lines of different EGFR expression status were used as target cells: 1) HCC827 cells, which contain high EGFR copy number amplification and 2) NCI-H460 cells, which contain zero EGFR copy number amplification. HCC827 and NCI-H460 cell lines were obtained from the ATCC® EGFR Genetic Alteration Cell Panel (TCP-1027™) and were cultured following recommended protocols using RPMI-1640 (Corning Cat. No. 15-040-CM) supplemented with 2 mM L-glutamine (Corning Cat. No. 25-005-CI) and 10% fetal bovine serum (FBS) (Corning Cat. No. 35-010-CV). All cell harvests were performed using Accutase® (Corning Cat. No. 25-058-CI). Cells were transduced using KILR Retroparticles for Adherent Cells (DiscoverX Cat. No. 97-003) following the manufacturer’s suggestions. Transduced cells were cultured under antibiotic selection with G418 (250 µg/mL for HCC827 cells and 500 µg/mL for NCI-H460 cells) for >7 days, and expression was confirmed using Total Lysis Control with the KILR detection reagents according to the KILR Retroparticles protocol recommendations. After confirmation of expression, the KILR transduced cells were scaled-up for assay.
KILR Cytotoxicity Assay
KILR retroparticle-transduced target cells were seeded in assay medium (RPMI-1640 containing 5% FBS and 2 mM L-glutamine) in 384-well spheroid microplates (Corning Cat. No. 3830) at 5×103 cells/well, and cultured in a humidified 37oC, 5% CO2 incubator for 48 hours for the formation of a single spheroid in each well. Spheroid microplates were sealed with breathable sealing tape (Corning Cat. No. 3345) prior to incubation to help prevent evaporation.
CAR-T cells were purchased from ProMab Biotechnologies. This study utilized EGFR scFv-CD28-CD3ζ CAR-T cells (ProMab Cat. No. PM-CAR1021-10M) which are engineered to target EGFR with a single chain variable fragment (scFv) and contain a CD3ζ antigen recognition domain and a CD28 co-stimulatory domain. Mock ScFV control CAR-T cells (ProMab Cat. No. PM-CAR1000-1M) were transduced with an empty vector. After target cell spheroid formation and incubation, EGFR and Mock ScFv Control CAR-T cells were thawed following the vendor’s recommendations and added in various effector-to-target (E/T) ratios ranging from 40:1 to 0.04:1 in assay medium using a CyBi®-Well Pipettor. Total Lysis Control from the KILR Cytotoxicity Assay kit was added to 3 wells of each cell type for final dilution of 1:20 in assay medium. Microplates were sealed with fresh breathable sealing film and cells were cultured for an additional 24 hours prior to luminescent readout.
To detect chemiluminescence signal, working detection solution was prepared by mixing the KILR detection reagents provided in the KILR Detection Kit (DiscoverX Cat. No. 97-0001M) following the vendor’s protocol and added to the microplates. Microplates were foil-sealed (Corning Cat. No. 6569), agitated on a plate shaker for 1 minute, and incubated for 1 hour at room temperature in the dark until luminescence was measured via plate reader. Percent luminescence was calculated by comparing luminescence from sample wells to luminescence from total lysis control wells.
Confocal Imaging
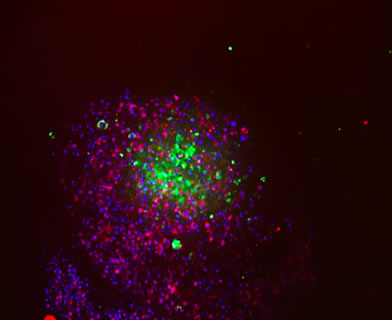
Confocal imaging of tumor cell spheroids was performed 24 hours after CAR-T cell addition. Briefly, cells were washed with Dulbecco’s Phosphate-Buffered Saline (DPBS) (Corning Cat. No. 21-031-CM) and fixed in 4% paraformaldehyde (Electron Microscopy Sciences Cat. No 157-SP) for 45 minutes. Cells were washed twice in DPBS and permeabilized with 0.2% Triton X-100 (Triton X-100 from Integra Cat No. T756.30.30) diluted in DPBS) for 1 hour. After fixation and permeabilization, cells were washed in DPBS and blocked with DPBS containing 2% BSA (Sigma-Aldrich® Cat. No. A9576), 5% FBS, and 0.1% Triton X-100 for 45 minutes. Cells were then stained with 10 µM Hoechst 33342 nuclei stain (Thermo Fisher Cat. No. 62249) and one of the following antibodies: 5 µg/mL anti-cytokeratin 7 Alexa Fluor® 488 (abcam Cat. No. ab185048), 5 µg/mL Alexa Fluor 488 isotype control (abcam Cat. No. ab199091), 8 µg/mL anti-human CD3 epsilon Alexa Fluor 594 (R&D Systems Cat. No. FAB100T-025), or 8 µg/mL Alexa Fluor 594 (R&D Systems Cat. No. IC002T). Cells were incubated overnight at 4°C and washed with DPBS prior to imaging on a Thermo Fisher CellInsight™ CX7 High-Content Screening Platform.
Results/Discussion
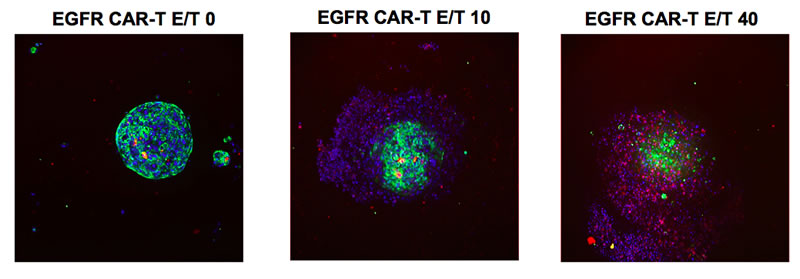
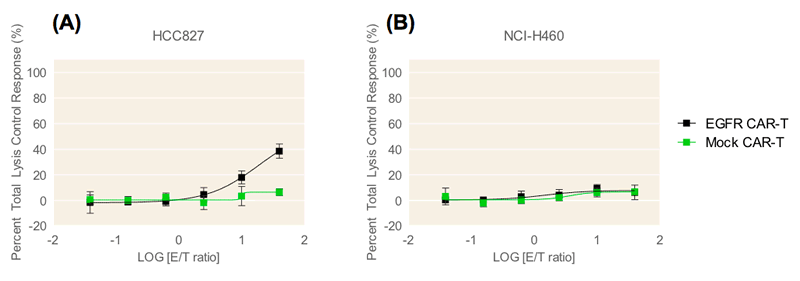
In this study, the cytotoxic effect of CAR-T cells on tumor cell spheroids was measured in a co-culture system in Corning spheroid microplates using the KILR assay. Two lung tumor cell lines from the ATCC® EGFR Genetic Alteration Cell panel, HCC827 and NCI-H460 cells, were transduced by KILR retroparticles for stable expression of a protein tagged with a β-gal reporter fragment that is released into media upon cell death. This enables the detection of target cell death via luminescence. The HCC827 tumor cell line contains high EGFR gene copy number amplification whereas the NCI-H460 cell line does not. CAR-T cells that target EGFR expressing cells (EGFR CAR-T cells) and mock control CAR-T cells engineered with an empty vector (Mock CAR-T cells) were used as effector cells against the two lung tumor cell lines which were cultured as spheroids. The EGFR CAR-T cells contain affinity tuned scFvs which exhibit higher anti-tumor efficacy to cells with higher expression of target receptor and no anti-tumor efficacy to cells exhibiting normal target receptor levels5. This affinity was confirmed by both confocal microscopy analysis and the KILR assay.
Confocal microscopy analysis reveals that the EGFR CAR-T cells encompassed and began to penetrate the HCC827 spheroid within 24 hours of CAR-T cell addition (Figure 1). As E/T ratio increased from 10:1 to 40:1, invasion of the CAR-T cells into the HCC827 tumor spheroid and subsequent tumor cell lysis was visible. Cell lysis was similarly detected using the KILR Detection Kit (Figure 2). EGFR CAR-T cells displayed dose-dependent targeting of HCC827 spheroids, with maximum 54% cell lysis at an E/T ratio of 40:1 (Figure 2). The EGFR CAR-T cells did not display detectable cytotoxic effects in the NCI-H460 spheroids which lack EGFR gene copy number amplification, demonstrating the EGFR amplification target specificity of the ProMab CAR-T cells. Target cell cytotoxicity was absent or minimally detected in both cell lines upon the addition of mock control CAR-T cells. This data supports that the EGFR CAR-T cell-induced cytotoxicity in HCC827 cells detected by the KILR assay is target specific.
Conclusions
Methods for testing different models of CAR-T cells in vitro against tumor spheroids could provide further insight into viable antigen targets before these models reach the clinical stage. The present study demonstrates the utility of the KILR Cytotoxicity assay combined with the spheroid microplate as KILR-transduced cell spheroids can be formed, cultured, and assayed directly in the same plate. Furthermore, cytotoxicity induced by second generation affinity tuned CAR-T cells against variable EGFR-expressing target tumor cell lines validates this model as a high-throughput compatible system for screening CAR-T cells against tumor spheroids.
*KILR is a registered trademark of DiscoverX Corp
Footnotes
-
1. Terry M. (2017, October 20). Why This Pharma May be the Best CAR-T Bet, Not Gilead. Retrieved from
-
2. Zhang BL, et al. (2016). Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci China Life Sci. 59:340-348.
-
3. Newick K, Moon E, Aldelda SM. (2016). Chimeric antigen receptor T-Cell Therapy for solid tumors. Mol Ther Oncolytics. 3:16006.
-
4. Xu, Xian, et al. (2014). Three-Dimensional In Vitro Tumor Models for Cancer Research and Drug Evaluation. Biotechnol Adv. 32(7): 1256-1268.
-
5. Liu, X, et al. (2015). Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75(17): 3596-3607.