A Xeno-free Culture System for hMSC Isolation and Expansion
Human mesenchymal stem cells (hMSC) are an important tool in regenerative medicine and cell therapies, as they have many attractive cellular characteristics. hMSCs present many advantages for therapeutic applications because they can be sourced from a wide range of tissue sources, are immune-privileged, and are able to migrate to tumors and wounds in vivo. Today hMSCs are one of the most common stem cell types found in Cell Therapy clinical trials.
In most clinical applications, hMSCs are expanded in vitro before use. In this case, the quality of the culture medium and its performance are critical, since hMSC properties can be significantly affected by medium components and culture conditions. In addition, Cell Therapy and tissue engineering applications have high requirements for safety and quality, including reducing the risk of any possible contamination from animal-derived components. Thus, culturing hMSCs in serum-free, xeno-free conditions is highly preferred.
Historically, finding an efficient way to culture hMSCs in a xeno-free system has been a challenge, as many common hMSC growth and expansion media still contain or require serum or other animal-derived components. In addition, commercially available xeno-free media designed for hMSC isolation are scarce.
In the poster, “A Xeno-Free Culture System for hMSCs from Various Sources Suitable for Initial Isolation and Expansion Toward Clinical Applications”, a novel xeno-free culture system for hMSCs was evaluated. The medium used in the study was NutriStem® MSC XF Medium, along with associated xeno-free materials for attachment, dissociation, and cryopreservation.
As part of this study, the NutriStem® MSC xeno-free culture system was evaluated for initial isolation of hMSCs from various sources and for long-term culturing under serum-free, xeno-free culture conditions. This system was designed to support the stringent requirements of clinical use.
Results showed that the NutriStem® MSC xeno-free culture system efficiently supported initial isolation and expansion of hMSC from various sources and effectively maintained hMSC features including:
- Typical fibroblast-like cell morphology
- Phenotypic surface marker profile
- Differentiation capacity
- Self-renewal potential
- Genetic stability
Immunophenotyping of hMSC-AT
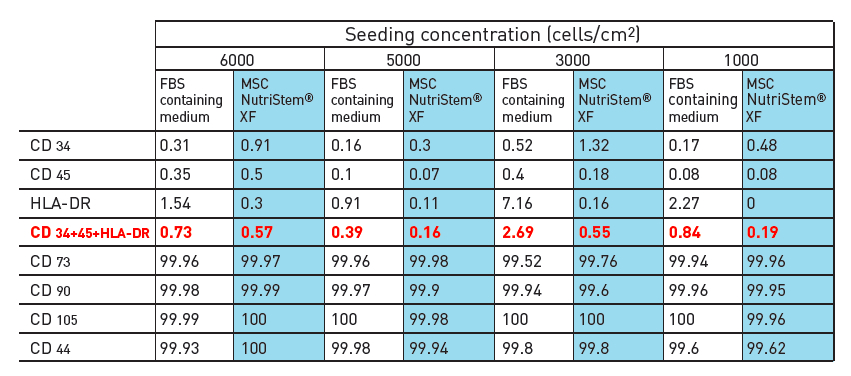
In addition to maintaining hMSC features, there were other advantages of using a xeno-free culture system over one containing FBS. For instance, a higher number of hMSCs were obtained after isolation as compared to media that contain FBS. Enhanced purity of the culture with a decreased amount of hematopoietic cell contamination was also found.
Evaluation of hMSC-BM isolation using MSC NutriStem® XF vs. FBS containing medium
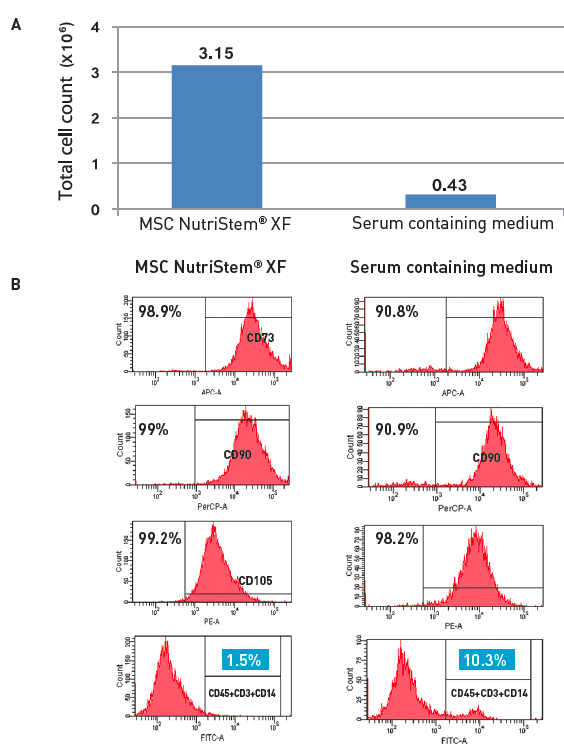
NutriStem® MSC XF Medium also demonstrated benefits over other serum-free systems with respect to the following:
- hMSCs can be isolated efficiently from various sources
- Achieved a higher proliferation rate compared to other commercially available serum-free media
- Supported long-term culture of hMSCs
- Cells retained essential hMSC characteristics
