
Accelerating the Cell and Gene Therapy Journey with Plug-and-Play Viral Vector Production Platforms
The demand for high-quality clinical-grade viral vectors has reached an all-time high, presenting a significant challenge for companies aiming to accelerate the development and potential commercialization of their cell and gene therapy (CGT) candidates. With the expanding range of approved therapies and their applications broadening beyond ultra-rare diseases, there is an urgent need for viral vector manufacturing technologies to evolve rapidly. Implementing scale-down models to develop and refine production methods that are predictive of conditions at a larger scale effectively reduce the financial burden of development, making it more accessible for developers. The current lack of standardized manufacturing procedures for lentivirus (LVV) and adeno-associated virus (AAV) vectors, commonly used in ex-vivo and in-vivo gene therapies, results in complexities and delays in achieving good manufacturing practice (GMP) compliance for crucial clinical trials.
Standardized plug-and-play manufacturing platforms could revolutionize viral vector production by effectively expediting product development timelines and meeting the surging demand for viral vectors. With templated process frameworks, these versatile systems offer a game-changing approach that not only simplifies and streamlines viral vector manufacturing but also allows for easy adaptation to the specific requirements of each CGT product. Thereby paving the way for faster development, streamlined manufacturing, reduced costs, and a stable and scalable supply of high-quality clinical-grade materials to ensure successful commercialization.
Accelerate Your Path from Gene to Clinic: Achieve GMP Goals in Half the Time with BravoAAV™ and ProntoLVV™
AGC Biologics, a global contract development and manufacturing organization (CDMO) has three decades of AAV and LVV development, manufacturing, analytical, and regulatory expertise, making them a key industry player to help establish standard procedures for large-scale viral vector manufacturing of these vectors in response to current challenges. AGC Biologics has successfully developed plug and play platforms for both AAV and LVV that can be easily adapted to different therapeutic genes of interest (GOI) without starting the development from scratch for each product, making the transfer to GMP as fast and smooth as possible.
Development of the BravoAAV™ and ProntoLVV™ viral vector platforms utilized the GFP transgene coupled with proprietary packaging plasmids. The GFP gene can then be exchanged for any therapeutic genes through a straightforward cut and sew cloning strategy. This ensures rapid development and by maintaining consistent upstream and downstream processes and analytics across different GOIs, companies benefit from significantly shortened timelines and a smoother, faster progression through process development as they advance their unique therapies towards GMP. Additionally, because the platform, processes, and protocols remain the same, the data gathered from one transgene can be applied for future therapeutic genes to expedite regulatory approvals and ease the path to commercialization.
Depending on the gene of interest, some fine tuning of the production process could be needed, and small-scale models are in place to set easily and rapidly make these adjustments. Furthermore, product specific analytical methods will be developed for each process.
Establishing a Platform Process
Upstream Process
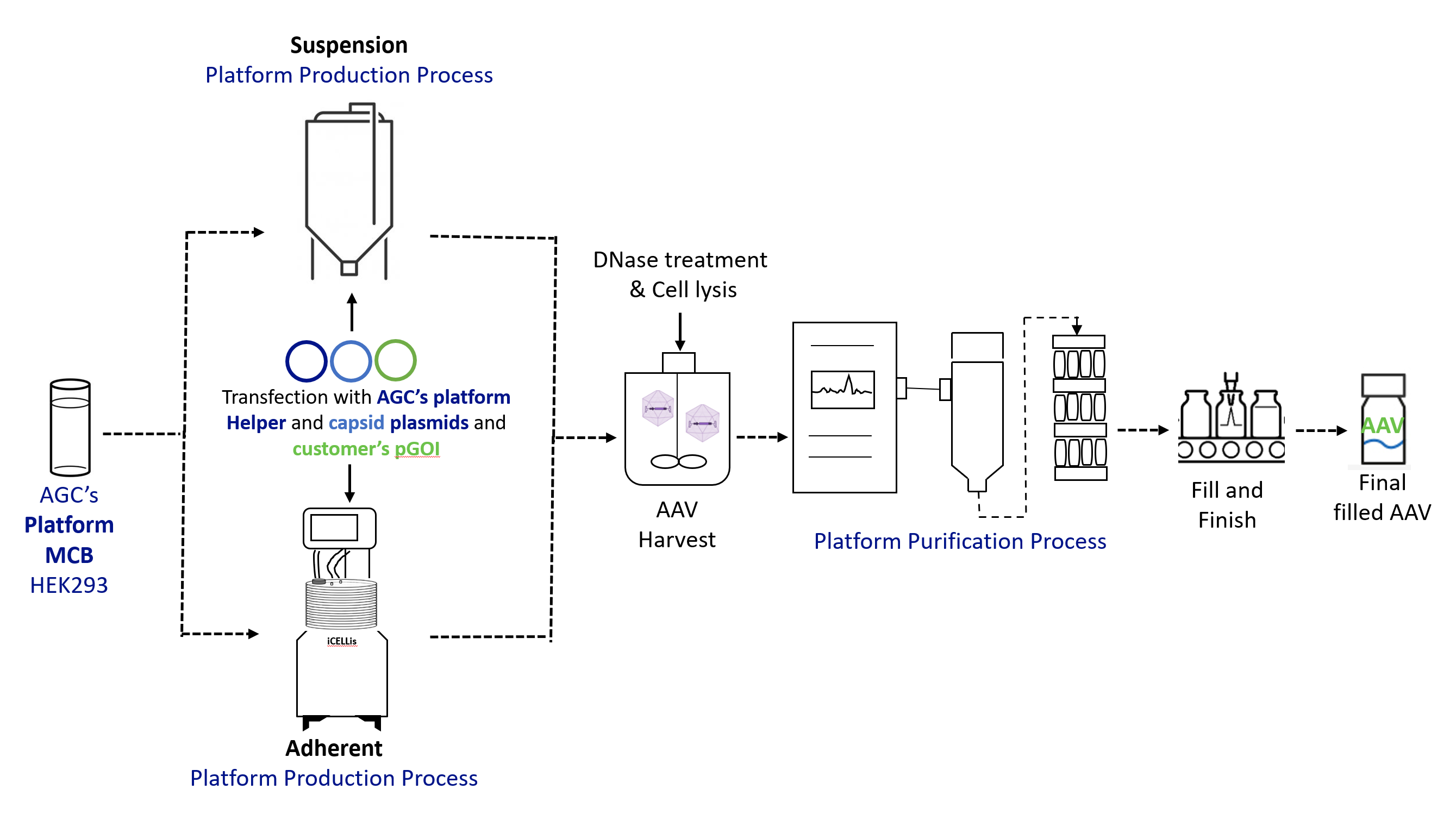
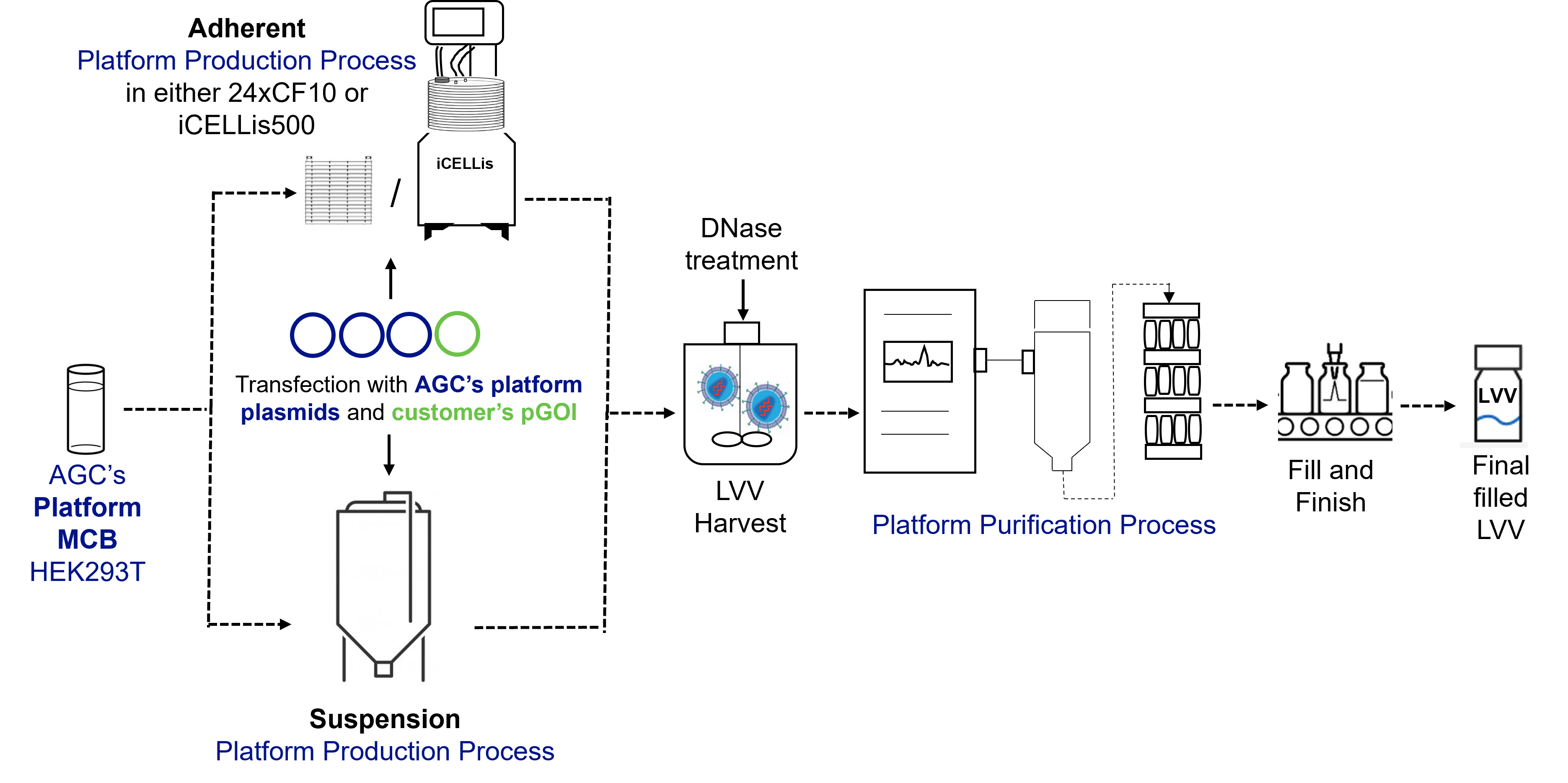
For both the BravoAAV and ProntoLVV platforms, the upstream (US) process is based on transient transfection of suspension HEK293 or HEK293T cells and was developed and optimized using the scale-down iCELLis® Nano packed bed bioreactor system for adherent and STR 3L bioreactors for suspension. Several different variables were tested at small scale to optimize the process including seeding cell density, culture medium, culture conditions, transfection settings, and harvest or lysis strategy.
Finally, the process parameters established at small-scale were validated on the full-scale iCELLis®500 system for the adherent, and in 200L STR bioreactor for suspension which confirmed good scalability and equivalent performance of the process.
The BravoAAV platform was developed with AAV6, known to be a challenging serotype, but it has been extensively tested with other secreted and retained serotypes to ensure that the optimized platform process is effective for different AAVs.
Downstream Process
Similarly, downstream (DS) parameters were established at small-scale with a focus on contaminant removal (i.e., Host Cell Proteins, Host Cell DNA/plasmid DNA, Benzonase and Bovine Serum Albumin (BSA, only for adherent processes)), while preserving vector infectivity to meet the highest quality standards.
For LVV the bulk vector after clarification is subjected to anionic exchange chromatography, concentration and diafiltration via tangential flow filtration (TFF), and finally, sterile filtration and filling.
For AAV, the DS process couples the capture step by affinity chromatography together with a polishing step to increase the full/empty particles ratio, a TFF-based concentration and formulation step. Several resins and membranes were evaluated to identify the best performing ones for both affinity and anion exchange (AEX) chromatography.
Overall, the yield of the optimized DS process ranges from 20% to 40%, with a percentage of full particles exceeding 80%. For LVV, the average process recovery after DS processing is approximately 30%.
The AAV and LVV platforms include:
- Ready to use GMP adherent and suspension MCB (Master Cell Bank) manufactured in-house
- Off-the-shelf packaging plasmid DNA (pDNA) manufactured in-house and supplied at different quality levels (high-quality and GMP-grade)
- Prequalified scale-down models to simplify Process Performance Qualification (PPQ) during process development and expedite the path to commercialization.
- Templated material approach allows to the transgene to be easily swapped while keeping the process and analytics constant for retained simplifies bill of materials (BoM) and helps ease regulatory burden
- 160+ in-house QC tests (95% performed in-house) for the characterization and release of LV and AAV with orthogonal strategies like viral genome, viral particles quantification, infectious viral titer, and process related impurities.
The BravoAAV and ProntoLVV platforms are suited for production at different scales for suspension and adherent cultures, allowing companies to select the most suitable scale for their specific production needs while maintaining process consistency.
BravoAAV Platform: adherent (200 L, 500 L and 750L) and suspension (50 L, 200 L, 500 L, 1000 L and 2000 L)
ProntoLVV Platform: adherent (48 L, 200 L and 500 L) and suspension (50 L, 200 L, 1000 L, and 2000 L)
With standardized and pre-qualified production and purification protocols, these platforms have demonstrated reliability across numerous transgenes to produce viral vectors with high potency and quality suitable for both ex-vivo and in-vivo applications. These platforms bring a new templated approach to complex AAV and LVV development and manufacturing, setting a new standard in the industry.
Global Partners for Successful Commercialization
With an established track record of success in the CGT field, AGC Biologics possesses a comprehensive understanding of the regulatory landscape and the entire development pathway. Notably, the global cell and gene team has successfully supported four commercial viral vector products, three commercial cell therapies and more than 30 CGT clinical trials across Europe and the United States. This expertise is complemented by a robust global network of manufacturing facilities and supply chain support across seven sites on three continents. These combined strengths allow the CDMO to offer comprehensive solutions and services from early stages through clinical and commercial stage manufacturing.
The addition of the standardized ProntoLVV™ and BravoAAV™ platforms demonstrates AGC Biologics‘ commitment to establishing best practices and manufacturing standards that will continue to benefit and propel the industry forward. By simplifying and accelerating the path to commercialization, these platforms allow companies to achieve clinical and commercial milestones more rapidly to deliver effective therapies that positively impact patient outcomes.
