Achieve Quality and Consistency With a Serum-Free, Chemically-Defined Workflow for T Cell Therapy
Chimeric antigen receptor (CAR)-T cell therapy has undeniably transformed modern medicine in the fight against cancer. This cell therapy uses patient (autologous) or donor (allogenic) T cells that have been modified to recognize and attack cancer cells expressing target ligand. The first CAR-T cell therapy was approved in 2017, and since that time, five others have been added to the list. However, to realize the full potential of cell therapies, we must overcome regulatory challenges regarding the quality, consistency, and sourcing of the raw materials and ancillary reagents required for those therapies.
In particular, ancillary cell culture reagents used throughout the CAR-T cell manufacturing workflow are critical to successful expansion, activation, and differentiation of T cells with the appropriate therapeutic profile. A recent webinar titled A Serum-free, Chemically-Defined Workflow for T Cell Therapy, presented by BioLegend scientist Dr. Zayda Piedra, PhD, discusses key components of a CAR-T workflow, current challenges, and emerging bioprocessing solutions that close the gaps for T cell therapy to ease transition from research to clinic.
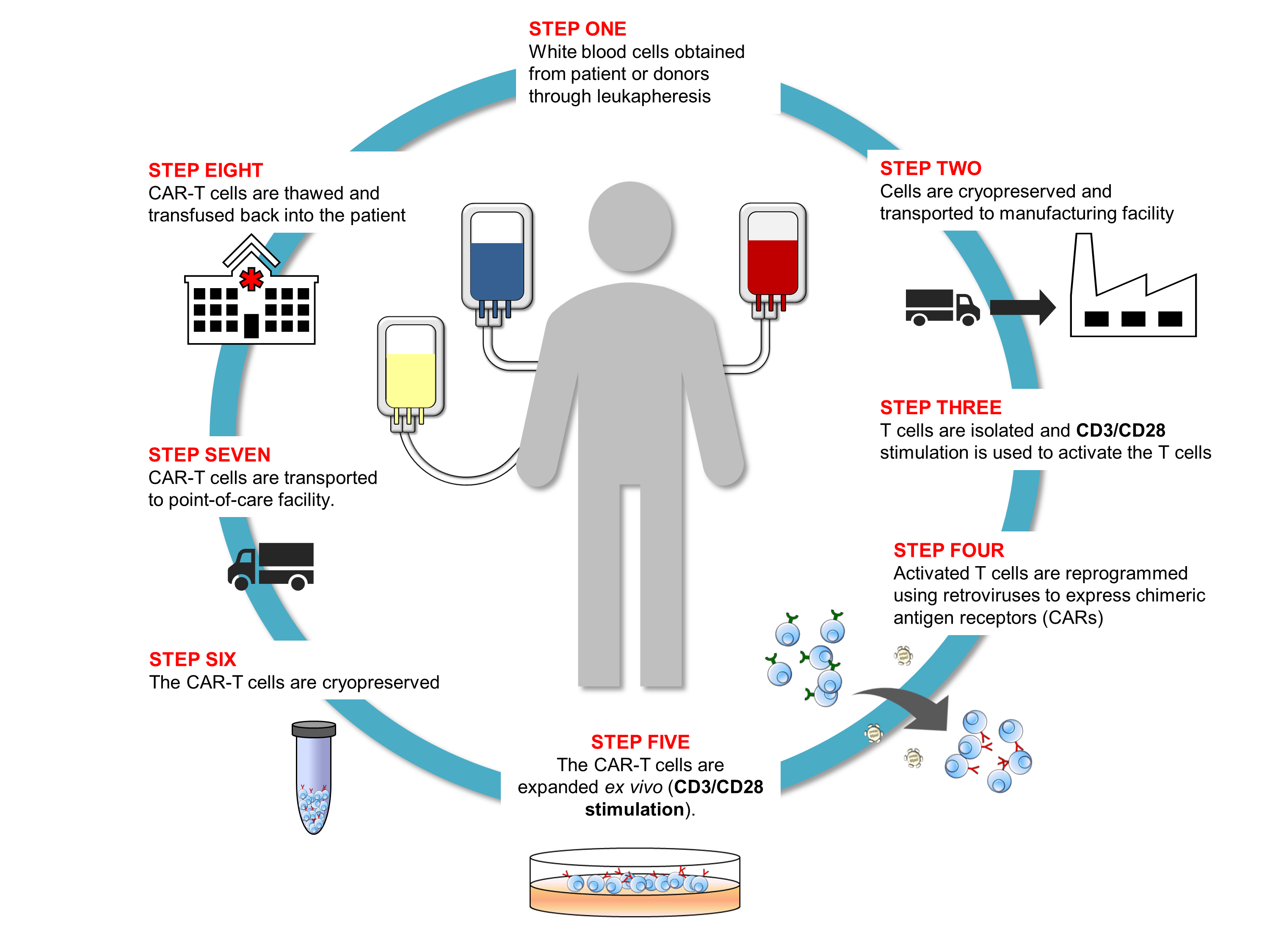
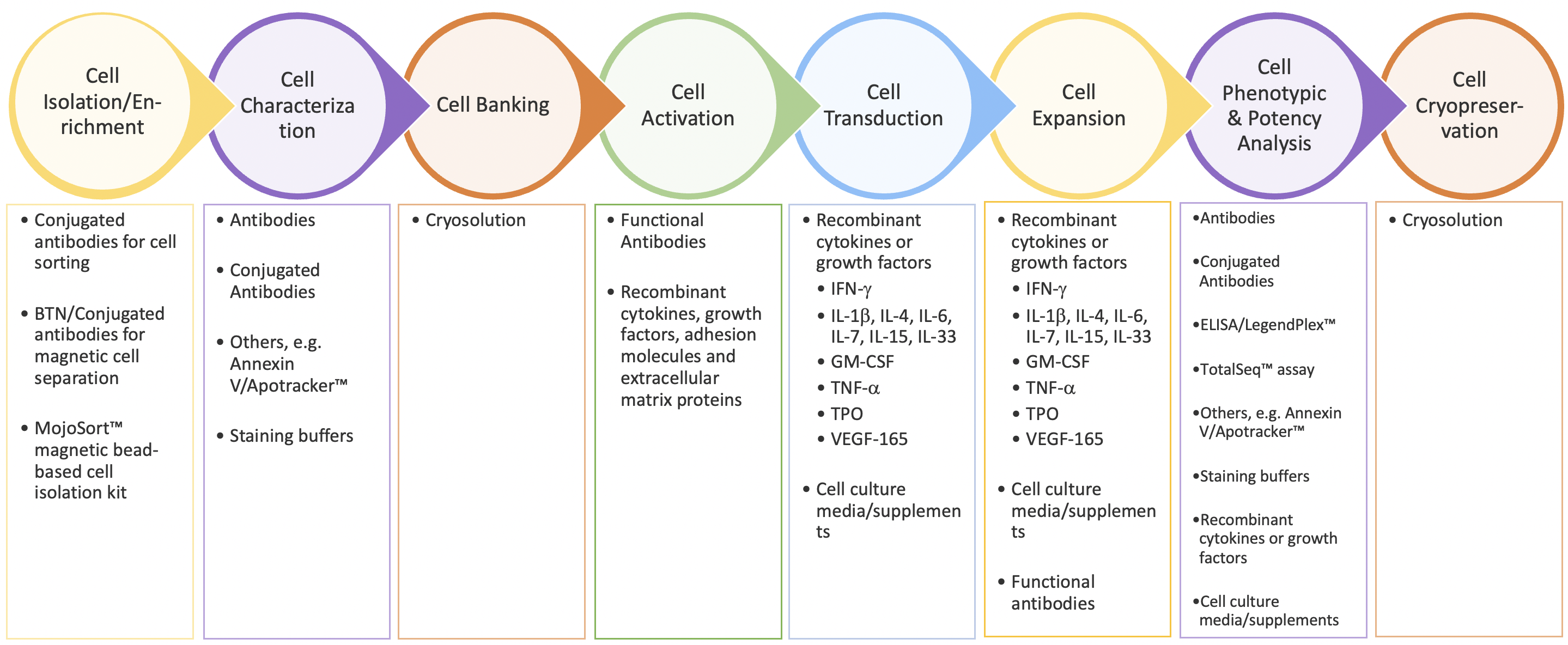
As Dr. Piedra emphasizes in her presentation, the CAR-T workflow is a complex, multi-step process that requires control and consistency throughout to produce batches of T cells with the desired characteristics (Figure 1). Because the cells themselves are the medicinal product, all of the bioprocessing reagents used in the workflow must meet GMP standards to ensure their quality, consistency, and safety for downstream manufacturing needs. BioLegend’s GMP-grade raw materials and ancillary reagents are manufactured in a dedicated GMP facility (ISO 13485:2016 and MDSAP certified) according to standardized processes and undergo extensive QC testing and QA review before release to support downstream CAR-T cell manufacturing workflows.
Disadvantages of Serum-Based Media
In conventional CAR-T workflows, manufacturers traditionally include serum or serum derivatives such as human AB serum for key cell culture and manipulation steps. While effective to grow cells, human AB serum poses significant drawbacks:
- Contains undefined components
- Lot-to-lot variability which requires pre-screening
- Performance is influenced by donor-specific characteristics (e.g., age, ethnicity)
- Potential to introduce pathogens into the workflow
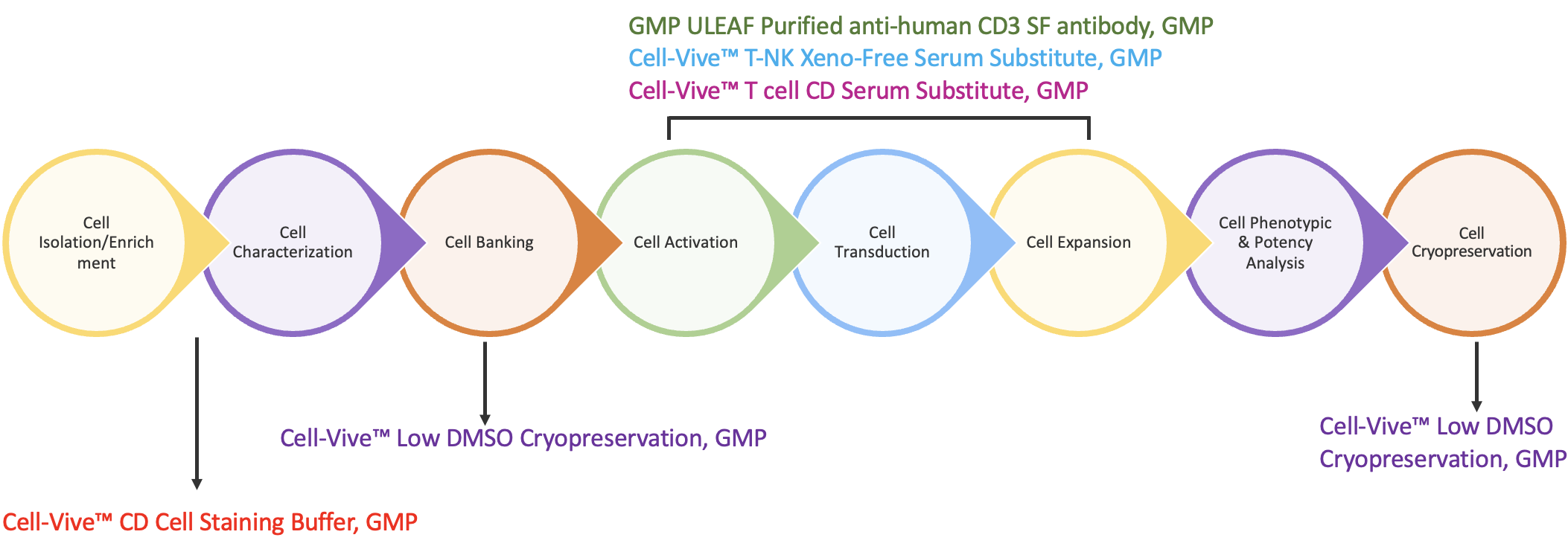
All together, these factors introduce variability and safety risks into the CAR-T workflow that are undesirable and costly. To overcome these risks, BioLegend developed GMP-grade serum-free and chemically-defined media supplements to take the place of serum-derived components at key manufacturing stages, including T cell isolation, activation, expansion, and cryopreservation (Figure 2).
T Cell Isolation
The first step in the CAR-T workflow is to isolate T cells from the heterogeneous peripheral blood mononuclear cell (PBMC) collection. Then ensure T cell population with Cell-Vive™ CD Cell Staining Buffer, GMP, a chemically-defined, antibody diluent and cell wash buffer prepared without human or other animal components. It is optimized for use in immunofluorescence staining protocols with high viability and low non-specific binding. To minimize safety risks, the buffer is tested to ensure low endotoxin levels and sterility verified by bioburden testing, making it ideal for T cell isolation protocols.
T Cell Activation
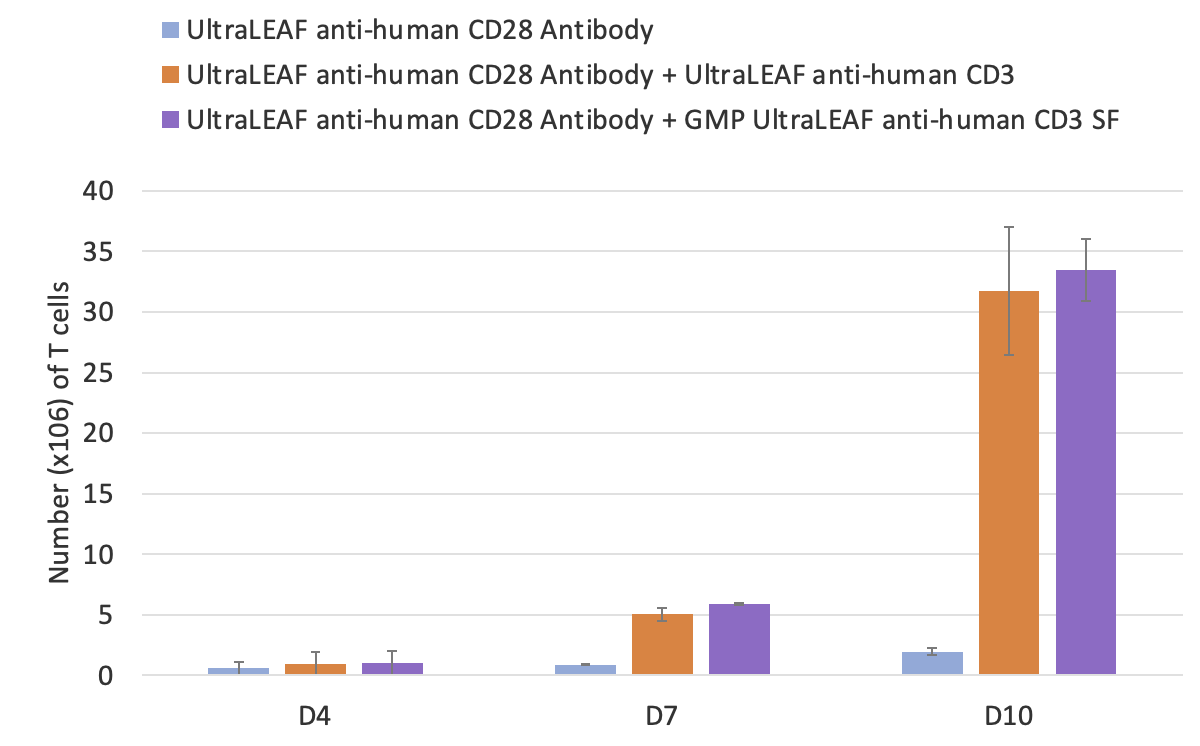
Once isolated and characterized, T cells are then activated with monoclonal antibodies like anti-CD3. BioLegend manufactures GMP Ultra-LEAF™ Purified anti-human CD3 SF Antibody (clone OKT3) under serum-free conditions without additional animal or human-derived materials. Dr. Piedra presented functional data showing the antibody is effective for T cell activation and expansion in ex vivo cell culture applications. PBMC-derived T cells were activated in the presence of Ultra-LEAF™ anti-human CD28 antibody alone or combined with either Ultra-LEAF™ anti-human CD3 or GMP Ultra-LEAF™ anti-human CD3 SF and cultured for a period of 10 days. The data show that the viability and the total number of T cells was comparable between the two CD3 antibodies (Figure 3).
T Cell Expansion
Following activation, expansion of the T cells is required to increase the total viable cell count prior to downstream activities including genetic modification. For this, BioLegend offers a choice of formulations. Cell-Vive™ T-NK Xeno-Free Serum Substitute, GMP is a xeno-free, serum-free formulation. Cell-Vive™ T Cell CD Serum Substitute, GMP, is chemically-defined and manufactured without animal and human components. This provides additional control, consistency and safety for T cell-based workflows. Both are formulated to support high PBMC-derived T cell expansion and cellular viability. They can be used in conjunction with the basal media of choice to support a variety of T cell activation methods, while doing away with the safety risks and lot-to-lot variability associated with human AB serum.
When tested against fetal bovine serum (FBS), human AB serum, and another commercially available serum substitute using a standard T cell expansion protocol, the Cell-Vive™ T-NK Serum Substitute maintained high cell viability (>90% throughout cell culture) and showed superior cell expansion compared to other conditions after 11 days of culture (Figure 4).
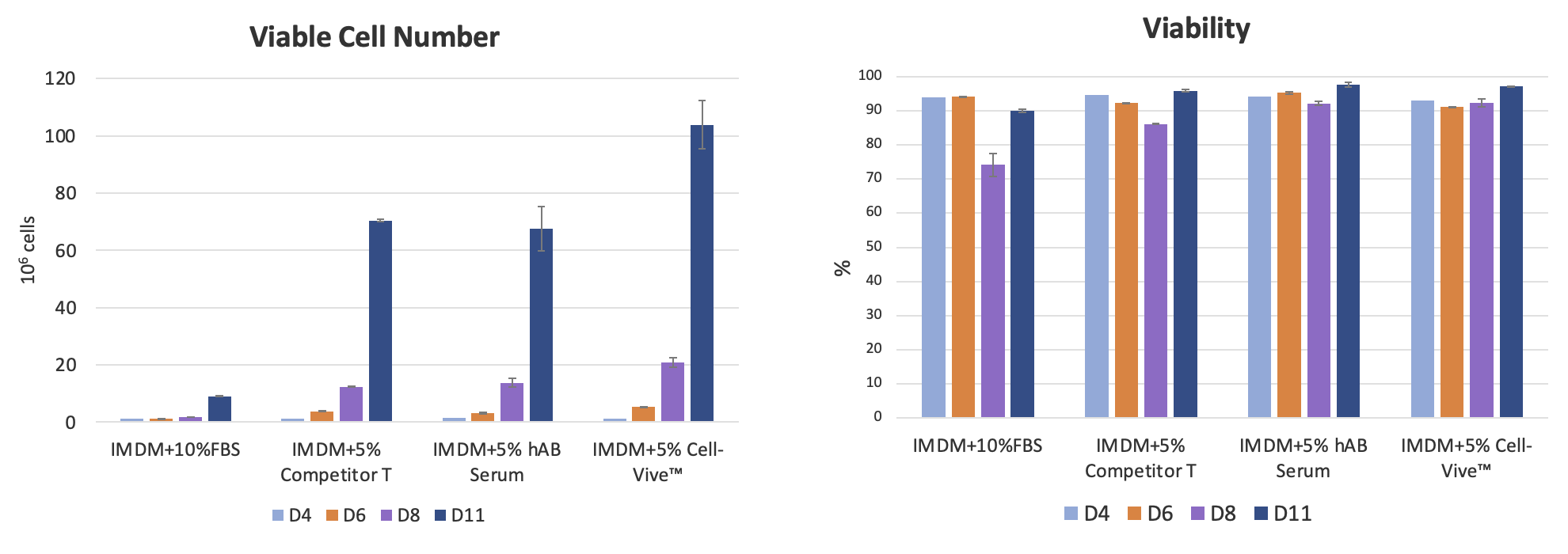
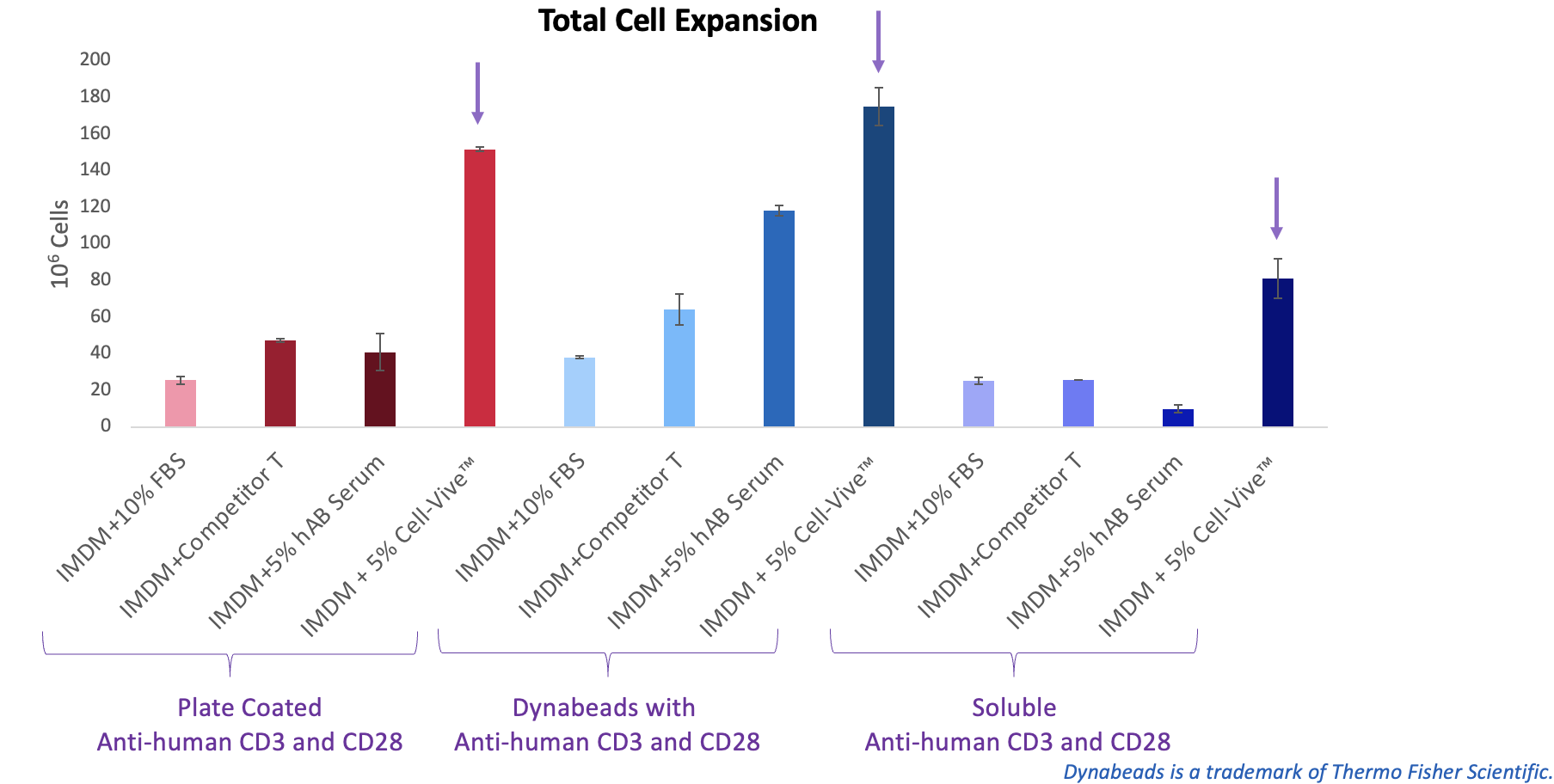
BioLegend also tested the serum substitute in combination with commonly used T cell activation methods—antibody-coated plates, Dynabeads®, or soluble antibodies—and found it supported more robust T cell expansion across all conditions (Figure 5).
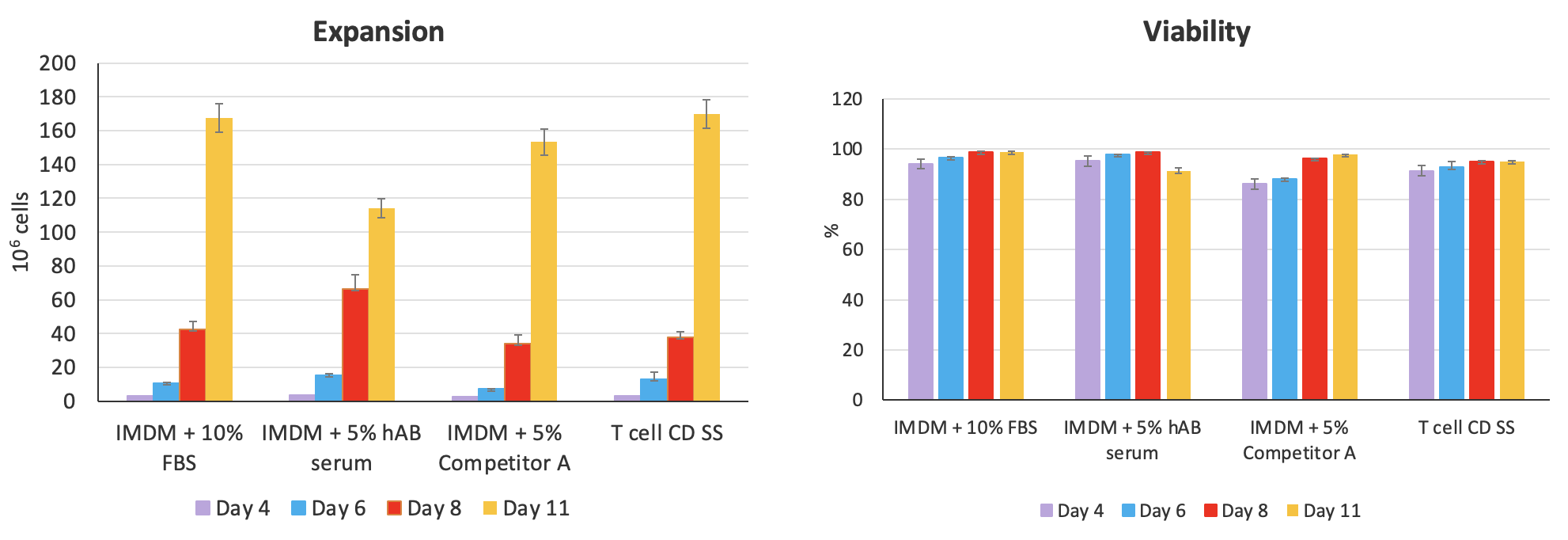
Next, Dr. Piedra presented data on the performance of the serum substitute for NK cell expansion before moving on to discuss the experimental results demonstrating the efficacy of the advanced chemically-defined Cell-Vive™ T Cell CD Serum Substitute, GMP. Comparative testing shows that the CD serum substitute supports high T cell expansion, preserves cellular viability, and maintains the ratio of CD4:CD8 at similar levels to FBS, hAB serum, or a commercially available equivalent product (Figure 6).
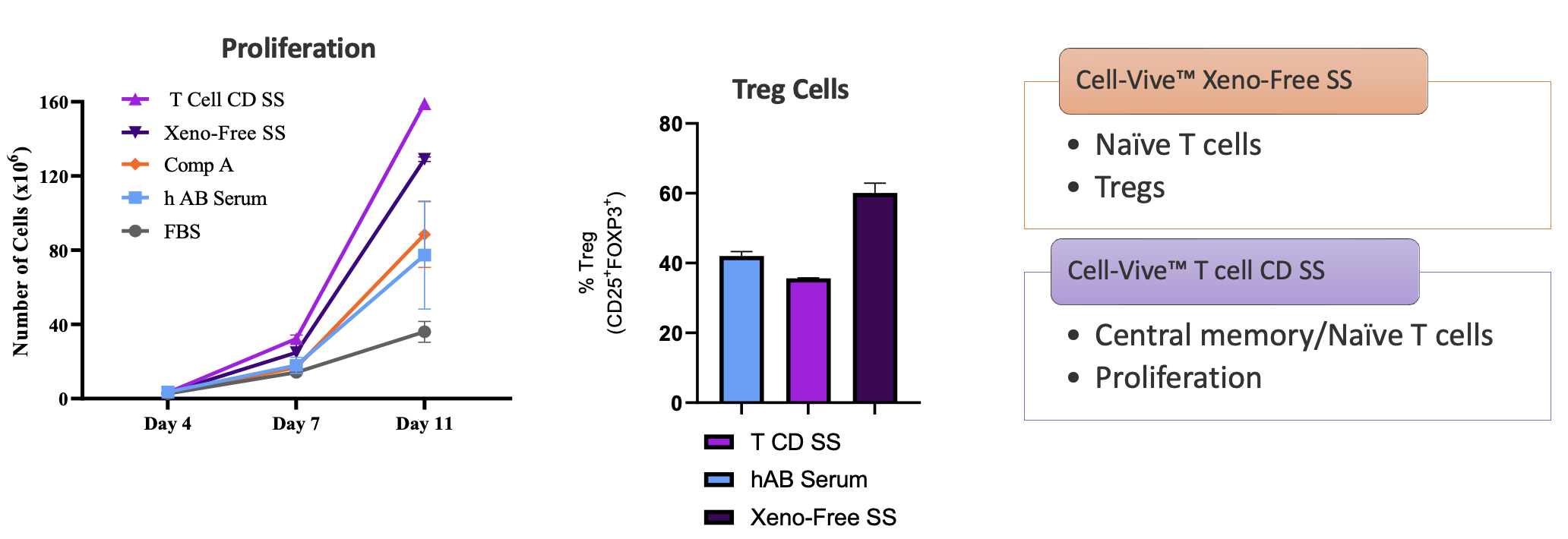
Interestingly, additional phenotypic characterization showed that the cells expanded in either the xeno-free or chemically-defined serum substitutes had different functional properties. Using the same protocol, the xeno-free serum substitute tends to expand a greater proportion of naïve CD3+ T cell and regulatory T cell (Tregs) populations, while the CD formulation preferentially expands central memory T cells and naïve T cells (Figure 7). End users can select the serum substitute formulation that works best for their application and desired cell type.
T Cell Cryopreservation
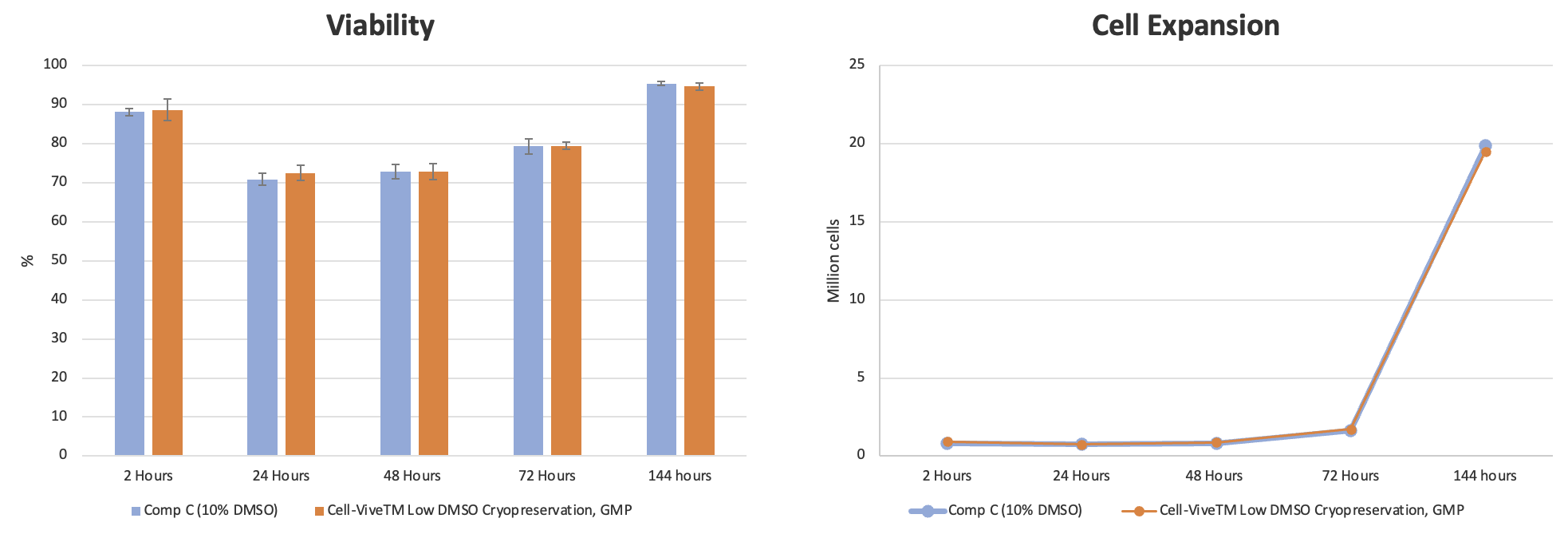
Preserving T cells is crucial at specific points in the process chain. First, the original tissue isolate can be stored and stabilized in a cryopreservation solution until processing can begin. Second, after cell expansion, cryopreservation ensures that important qualities of processed cells are maintained for downstream applications. For these critical steps, BioLegend has developed a GMP-grade formulation with a low DMSO concentration to support cryopreservation of T cells from multiple sources (PBMCs, CD3+, expanded T cells) and minimize the negative effects of DMSO. Cell-Vive™ Low DMSO Cryopreservation, GMP is a protein free, chemically-defined formulation, prepared without animal components, that preserves T cell viability and expansion capacity post-thaw.
Experimental data shows that cell viability and percentage of T cell recovery remains high post-thaw and are at similar or higher levels compared to other commonly used cryopreservation solutions containing 10% DMSO (Competitor C) (Figure 8). Additionally, the proliferative capacity and ratio of CD4:CD8 T cells post-thaw are retained after cryopreservation using the Cell-Vive Low DMSO Cryopreservation, GMP solution (data not shown).
In closing, Dr. Piedra notes that while her presentation focused on BioLegend’s novel serum-free and chemically-defined GMP reagents, the company boasts a wide range of non-GMP solutions that can also be implemented across the T Cell therapy workflow to seamlessly transition from R&D to clinical applications. BioLegend also offers custom GMP development and manufacturing services for antibodies and ancillary reagents based on customer-specific program requirements.
We’ve only provided a brief summary of Dr. Piedra’s webinar here, so be sure to view the webinar in its entirety to get specific details on the experimental protocols and analytical testing data featuring BioLegend’s suite of GMP reagents for cell therapy manufacturing.
Drug Master Files (DMFs) will be available for all GMP cell culture reagents