Bio-Techne’s GMP ProDots Proteins™: Closing the Loop in Cell Therapy Manufacturing
As cell therapy manufacturing continues to grow, patient safety is at the core of developing robust and efficient manufacturing processes. The ability to close the manufacturing process mitigates the risk to the final therapeutic and ensures an efficient, de-risked, cost effective process. To close the gap on the addition of critical cytokines into the process, Bio-Techne has developed GMP ProDots™ Proteins, lyophilized spheres of protein inside single-use sterile, weldable bags that can be directly integrated into closed process systems.
Reduce Risk in the Process with GMP ProDots Proteins
GMP ProDots Proteins are manufactured under rigorous quality processes to ensure the highest grade protein, increasing consistency between lots and supporting a continuous supply chain. ProDots Proteins are both efficient and compatible with automated systems. Each single-use bag of GMP-grade proteins is composed of an animal-free biofilm and carries two ports to allow for easy extraction: a needleless valve and a PVC tube that can be sterile welded directly into a manual or automated system. Limited user intervention allows for an efficient, consistent process that strengthens an existing closed process system.
What do ProDots™ Proteins look like?
Through Bio-Techne’s proprietary process, they have developed proteins as lyophilized spheres containing 5 µg of protein each. The spheres are held in an animal-free E-BEAM irradiated steril bag. The bags contain a defined number of spheres that reconstitute rapidly into culture media. Each bag can hold up to 25 mL of solution to completely dissolve each ProDots sphere. The reconstituted proteins are stable for at least 2 years in the un-reconstituted state.

Consistency
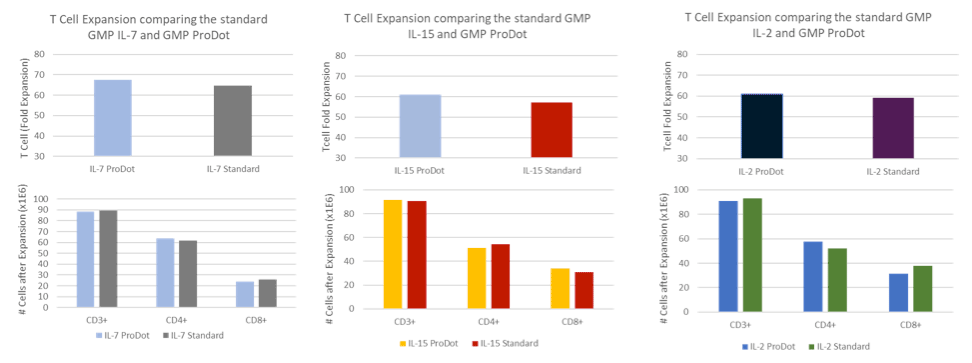
Supplying a consistent raw material that ensures expected results every time is at the core of Bio-Techne’s protein development. Their standard formulation of GMP-grade proteins is used for manufacturing the ProDots Proteins and they observed equivalent activity during T cell expansion. These results highlight the ease of transitioning from GMP-grade or research proteins directly into ProDots™ Proteins.
Customizable
Every cell or gene therapy process is different and Bio-Techne works with each customer to optimize their order to fit their process. Whether that is changing the amount of protein within each bag, or the cytokine mass in each dot, their team of custom specialists guides users to the optimal solution.
To learn more about ProDots™ Proteins and Bio-Techne solutions, please visit GMP ProDots™ Proteins