
Cell Flask Adapters Can Streamline the Cell Culturing Process
A Guest Blog by Randy Lockner, Product Manager, Beckman Coulter
Introduction
During various stages of the cell culturing process, centrifugation is frequently used to isolate extracellular products or to separate cells from their aqueous environment. Traditionally, cells and media are first transferred from a cell culture flask to a 15 mL or 50 mL conical tube before centrifugation. These transfer steps require operator labor and time, as well as the cost of the transfer vessel, and also introduce the potential for contamination during transfer.
With cell culture flask adapters,* however, the culture can be centrifuged directly in the flask. This helps streamline the cell culturing process by decreasing time and labor. Moreover, it reduces the potential for contamination and decreases overall labware costs.
Because these reusable adapters are made of a single piece of highly durable elastomeric material, they can withstand the rigors of use (e.g., centrifugal force, cleaning and sterilization) in the bioresearch laboratory.
When to Use Cell Culture Flask Adapters
Cell culture flask adapters support cell culture flasks during centrifugation, which is used in the cell culturing process (seeding, passaging or harvesting) and in studies of cellular products and activities (e.g., organelles, proteins, antibody production). During various phases of the cellular study, such as cell cycle, adhesion, motility and signal transduction, centrifugation can separate or concentrate cells from culture media and/or collect extracellular products.
Traditionally, the cells and media are transferred from a cell culture flask to another container—typically a 15 mL or 50 mL conical tube. After centrifugation, a cell pellet is either resuspended and transferred back to the cell culture flask for continued growth, or it is extracted for storage or downstream analysis. These transfer steps can be eliminated when culturing and centrifugation are carried out in the same flask—a protocol made possible with a cell culture flask adapter. This completely prevents the potential for contamination that can occur when cells and media are transferred to and from a tube.
Because they’re made of a single piece of highly durable elastomeric material, cell flask adapters can withstand the rigors of use in the bioresearch laboratory.
Use of cell culture flask adapters is appropriate for a variety of cell culture types, including adherent, suspension, hybridoma and primary cells. Some small and less-dense cells have been shown to seed better when centrifuged in the cell culture flask. Users of these adapters have also reported faster detachment of adherent cells during cell recovery. In addition, adapter use typically creates softer cell pellets that can be more readily resuspended.
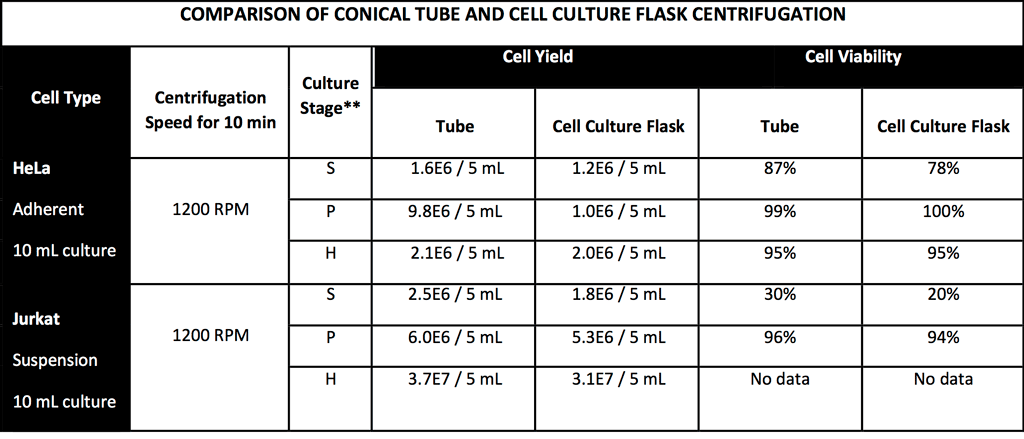
It is noteworthy that the multiple advantages provided by cell flask adapters are obtained without compromising results. Data illustrate that cell yield, cell viability, and endpoint analysis results are comparable whether cell cultures are processed traditionally or centrifuged directly in the flask using a cell culture flask adapter.
Adapter Use Delivers Comparable Results
Comparable results (Table 1) were obtained whether cell cultures were processed traditionally or centrifuged directly in flasks using a cell culture flask adapter. Specifically, data showed:
- Comparable cell viability
- Comparable endpoint analysis results
- Viral PFU counts
- Monoclonal antibody production
- Cytokine protein measured by ELISA
- Fluorescence detection of caspase from an apoptosis study
- Comparable cell yield
For optimization of cell yield, cell culture flask adapters can be centrifuged at speeds greater than those typically run in a 15 mL or 50 mL conical tube for cell concentration. This process can be performed without disruption of cells.
Table 1:
Using Cell Culture Flask Adapters
Beckman Coulter Cell Culture Flask Adapters are designed to allow centrifugation of T-75† (75 cm² area) or T-25† (25 cm² area)

cell culture flasks in Beckman Coulter Allegra X-14R, Allegra X-15R and Allegra X-12 Series centrifuges using SX4750 and SX4750A rotors.
Before first use and after cleaning the adapter, it is important to lightly coat the inside of the bucket with dry release agent (Beckman Coulter Life Sciences P/N 392819) to facilitate the removal of the adapter from the SX4750 or SX4750A round bucket.
Filled flasks can be easily inserted and extracted from cell culture flask adapters before and after centrifugation. Easy extraction of the flask from the adapter helps ensure minimal disturbance of the pellet.
Expect a Faster Spin
To optimize cell yield, higher centrifugal force (up to 3,000 rpm/2,000 x g) can be used when centrifuging flasks in cell culture flask adapters. The maximum allowable centrifugal speeds for cell culture flask adapters designed for Corning® T-75 and T-25 flasks vary depending on working volume.
The adapter for T-75 flasks can be centrifuged up to 3,024 rpm (2,001 x g) with 50 mL (a commonly used culture volume), but lower speeds are required for higher or lower working volumes. At up to 25 mL, the adapters for T-25 cell culture flasks offer superior performance at 3,200 rpm (2,000 x g).
Various types of cell cultures (adherent, suspension and primary tissue) have been shown to maintain good cellular function and good viability (> 90%) when spun at higher forces than those used for traditional processing.
The Pellet Will Look Different
The difference in geometry between the flat bottom of the flask and the conical tip of the tube results in a different appearance of the cell pellet. When centrifuging the flask directly, the cell pellet is softer and spreads more loosely over the bottom of the flask. Although the appearance of the cell pellet is different, the results obtained from centrifuging in the flask have been comparable to those from traditional methods (Table 1).
In addition, the separation line between the cell pellet and supernatant is less clearly defined. After centrifugation, users should expect to retain a thin layer of residual media when removing the supernatant. More spent media or washing buffer will be carried over to the next culture. This can dilute the strength of culturing nutrients, but that can be corrected with a smaller culture volume and increased centrifugal speed.
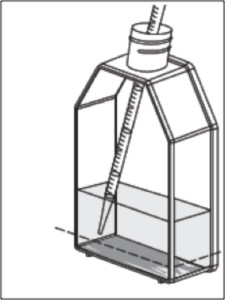
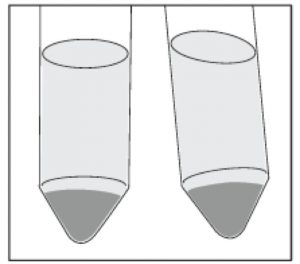
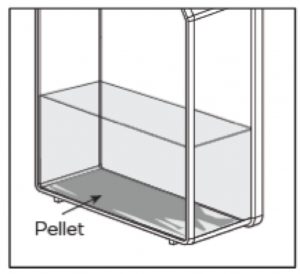
When centrifuging cells and media directly in the flask rather than in a conical tube, the resulting cell pellet is softer and spreads more loosely over the bottom of the flask.
For more information, including additional data comparing use of cell flask adapters with traditional methods, click here.
*Beckman Coulter Cell Culture Flask Adapters have a patent pending.
†Adapters currently available for Corning 75 cm² canted-neck cell culture flasks (P/N 430641U) and 25 cm² canted-neck cell culture flasks (P/N 430639).
Learn more at Beckman.com/centrifugation