CHI Oligonucleotide and Precision Therapeutics: Meeting Impressions
OPT Congress is in its 8th year of presenting sessions on Oligo Discovery & Delivery and CMC & Regulatory, and this year introduced a third session on Emerging mRNA Therapeutics (Congress). The congress consisted of 2 days of more than 55 scientific presentations, interactive discussions, and began with a short course on the Safety & Toxicity of Nucleic Acids. With a choice of Boston in-person or virtual attendance at an early-bird commercial delegate rate of $1699, I found it an unusually good value.
The scope of the conference included drug design, delivery, performance, and marketing− intended for researchers, developers, CMC and regulatory specialists, and technology suppliers. This meeting bridges the gap between those focusing on academia and those focusing on industry. CHI offered participation via a virtual platform including both live and recorded sessions, but I attended in person at the Revere Hotel Boston Common (Venue). I’d never visited this hotel previously but discovered that it was very close to, e.g., Boston’s Theatre District and Copley Square.
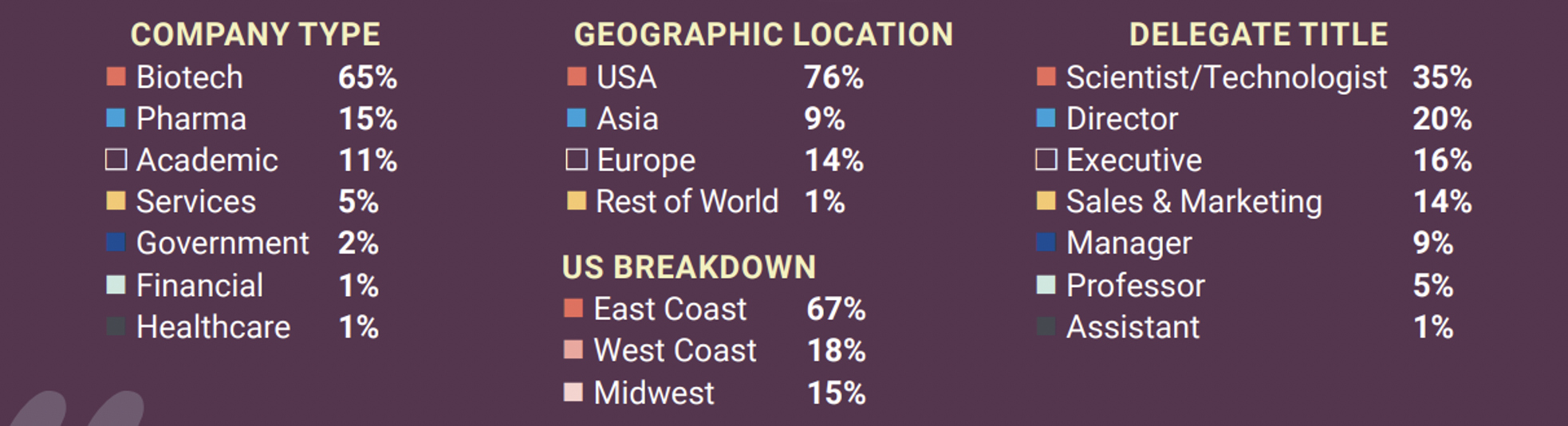
The speaker bios reveal a full spectrum of vitae from top university, pharma, and suppliers (Speakers). The program highlighted two days of both plenary and session presentations considering the science, business and regulatory aspects of many therapeutics and vaccines. My interest was in technologies supporting manufacturing and the molecular aspects of product design, including their structure, splicing, and editing− as well as supporting such functions as their targeting and silencing (Brochure). The organizers report the audience of approximately 300 attendees and, as representing a vendor of consulting services, I especially appreciated how open CHI is with their past attendee demographics:
Day One, Mon 13 Mar
The day began with a pre-congress short course on Safety and Toxicity of Nucleic Acids. Following opening remarks, the congress proper moved directly into their three separate sessions. CMC and Regulatory Strategies opened with Lubomir Nechev, Alnylam Pharmaceuticals presenting Developing Manufacturing and Quality Control Strategy for siRNA Therapeutics. He introduced the more successful manufacturing and quality control strategies for synthetic siRNA therapeutics, in particularly those delivered IV by LNPs, and sub-Q using GalNAc conjugates. I especially enjoyed his review of the production and justification of the GalNAc succinate for 3’ end conjugation in the drug product. He emphasized Alnylam’s ability to justify the use of this GalNAc succinate as a starting material is through an extensive impurity profile. The key is understanding and defining the fate of the worst-case levels of reactive and non-reactive impurities, such as protecting groups, solvents, etc. in the drug product production.
Day Two, Tue 14 Mar
A paper in the Discovery and Delivery session that had particular import to me was Using Computation to Enhance Target ID in RNAi Drug Discovery by James Longden, e-therapeutics, PLC.
He began with an overview of how the orchestration of data, computation, and biology can aid in the discovery and development of RNAi medicines. He then explained how e-therapeutics uses mechanistic network biology models to interrogate disease process complexity, test therapeutic hypotheses, identify novel targets, and finally to design effective nucleotide sequences. He demonstrated how they mine an extensive knowledgebase of hepatocyte-relevant data using deep learning to guide their GalNAc-siRNA technology in designing targeting solutions.
The new Emerging mRNA Therapeutics session featured papers on exciting new approaches in the molecular design and therapeutic targeting of RNA, but one paper of interest to me was Lipid Nanoparticles for Overcoming Biological Barriers to mRNA presented by Michael Mitchell, University of Pennsylvania. Unfortunately, a storm cancelled his flight, but CHI was able to quickly support his delivery virtually. He discussed the development of their nanoparticles for the in vivo delivery of various oligo’s. LNPs are popular because of their targetability and endonuclease protection. Their development of a pH driven release of cytosolically ionizable LNPs was very interesting. It provides such value as reduced cytotoxicity while enabling efficient release of active agent within the cell. He reviewed their combinatorial chemistry to design the ionizable lipids, orthogonal DOE to design the lipid/polymer formulations, and microfluidics to produce the actual LNPs.
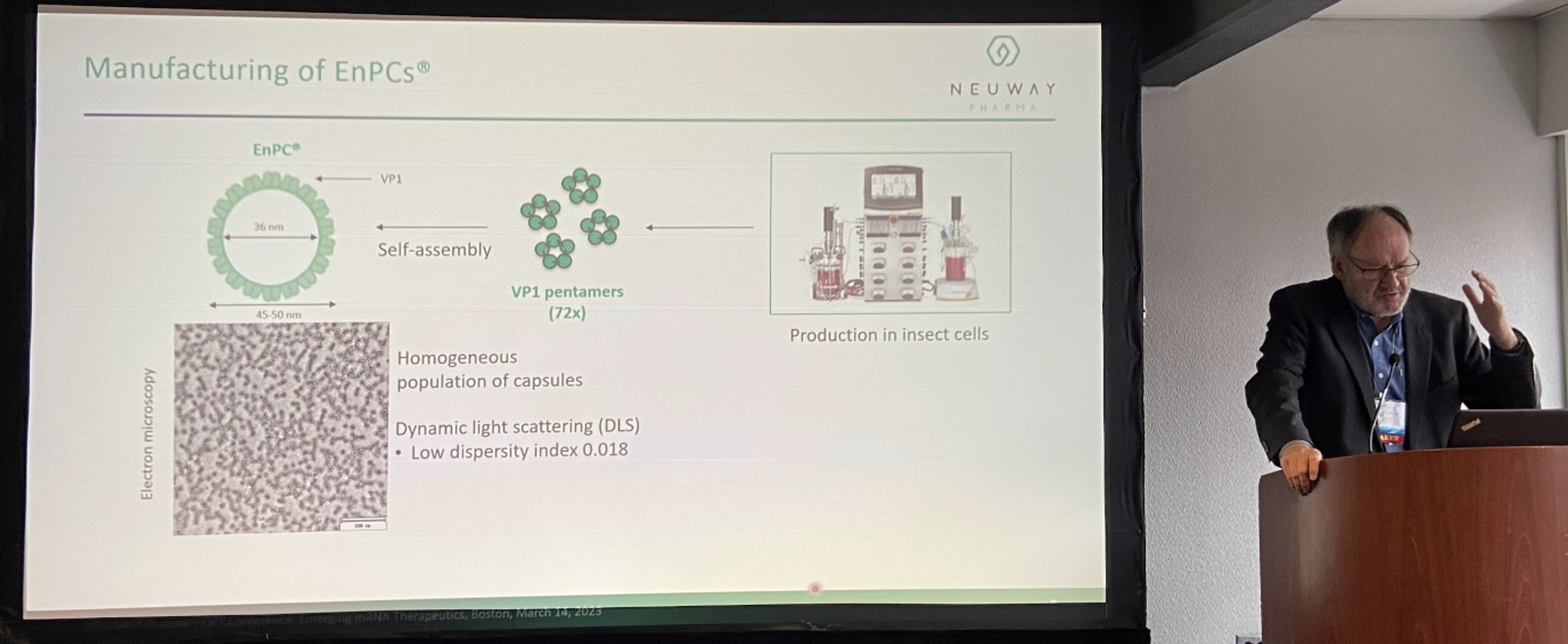
Figure: Ekkehard Leberer, ELBIOCON, presented on a NEUWAY technology in his lecture Therapeutic RNA Delivery to the Brain Using Protein-based Nanocapsules
Plenary presentations
Beyond the 3 individual sessions there were plenary presentations such as Steve Pascolo, University Hospital of Zurich and Founder & CEO, Miescher Pharma, presenting Development of mRNA-Based Vaccines. He reviewed the present, past, and future of mRNA vaccines including new mRNA formats (circular, replicating) and formulations (lipoplexes and polyplexes).
Breakout Discussions
There were breakout discussions for each of the three sessions, such as by June Park, siRNAgen, who led an interactive dialogue on Therapeutics led Oligonucleotide Discovery and Delivery. This considered such topics in emerging oligo technologies as AI/machine learning, challenges in the adoption of innovative technologies, and what’s next in oligo discovery and delivery.
Conclusion
I found the program to be comprehensive and the experience very comfortable and collegial. I especially appreciated the organizer’s ability to effectively support last minute adjustments of the schedule due to the winter storm.