CHO Media Development and Optimization – A look at three case studies
CHO media development and optimization is always a popular topic since media plays a key role in biomanufacturing success. In my BPI East coverage last month, “Biomanufacturing CHO Media – A look at different approaches and optimization opportunities,” I discussed different media development and optimization strategy options, highlighting decisions that companies need to make when thinking about how they will handle their CHO media development from scale up through commercialization of product candidates. These decisions must take into account many factors including, yield, product quality, timelines, impact on downstream purification, regulatory compliance and raw material sourcing, just to name a few. Companies may choose to develop their own platform media for their products, use an off the shelf media, partner to create a custom formulation or optimization, or take a hybrid approach.
Advantages of Partnering for Media Development and Optimization
When employing media development services, companies’ work closely with their media partners to provide them with the cell line, any special characteristics, process requirements, desired product quality, manufacturing equipment information and any other pertinent information. The media development company then utilizes their team of experts and equipment to test many different media combinations.
Since media development companies have all these competencies and this is their primary focus, they are typically able to fulfill timelines for media development much quicker than in-house development. Partnering is an attractive option for companies that want to have expertise and experience without developing those competencies in-house.
I recently came across a webinar, “Achieving Optimal Monoclonal Antibody Titer and Quality through Cell Culture Media Optimization,” presented by James W. Brooks, Ph.D., R&D Manager, BD Biosciences – Advanced Bioprocessing. I thought Dr. Brooks did an excellent job describing the option of outsourcing media development and optimization in more detail and presenting three representative case studies. The webinar begins with a discussion of the evolution of cell culture media and the shift in focus from getting the highest titers possible to now also looking further at the effect media has on protein quality.
Dr. Brooks then presents the case studies, which each addressed different biomanufacturing media problems, how the problems were approached, testing methods, and ultimately how a solution was reached. These case studies provide a good example of the kind of partnering that happens when outsourcing media solutions. At the end, Dr. Brooks described the various outsourcing options and also associated timelines, which provided good information for those considering media optimization or development services. I have summarized my highlights from the webinar, but please see the full webinar, available on demand, for more details.
Evolution of Media to Meet Industry Demands
Dr. Brooks started the webinar with a brief background on the history of mAb therapeutics manufacturing and associated challenges. He described how media has evolved to address these concerns, for example as a result of contamination risk concerns, there was a movement to remove animal derived components from media. As the focus has shifted throughout the years, media and supplements have been modified to meet changing industry demands. He went on to discuss that the most recent evolution is a media focus that has shifted from getting the highest titers to titers that are “good enough” with increasing emphasis on medium’s effect on protein quality and consistency. This is critically important in biosimilar development as “biosimilar approval requires demonstration of no analytically or clinically significant differences and that the biosimilar demonstrate similar pharmacokinetics as originator molecule.” Suboptimal media and supplements can impact both quality and performance.
Key elements of protein quality as described by Dr. Brooks included:
- Structure
- Glycosylation
- Charge Variants
- Aggregation
- Host Cell Impurities
For the purposes of these case studies, Dr. Brooks focused on glycosylation and charge variants. Glycosylation is determined by post-translational mechanisms of the cell and varies by cell line. Charge variants result from events during cell culture production and need to be minimized in final product.
Case Studies:
Case Study #1 – Issue: Acceptable cell growth and productivity with unacceptable charge variant profile.
In the first case study, Dr. Brooks explained a company developing a biosimilar IgG approached BD for optimization of an original medium. The original medium produced acceptable cell growth and IgG production, but an unacceptable charge variant profile. The company had screened multiple commercial media and found one that would produce an acceptable charge variant profile, but the new medium resulted in protein production and cell growth lower than the original medium. Consequently, the company came to BD Biosciences with the goal of enhancing the original media formulation to meet the desired charge variant profile while retaining cell growth and production.
BD’s approach was to modify multiple components of the original media. They needed to reach a peak viable cell density (VCD) of ≥ 5×106 cells per mL and production of ≥ 75% of the original media.
After some initial modifications of peptone and chemically defined (CD) components, 4 media met the criteria for VCD and productivity. BD then took these 4 media forward to look at the charge variant profile. Two of these media resulted in IgG with acceptable charge variant profiles. The end result was that two candidate media were successfully developed which provided the consistent protein quality profile, while maintaining required growth and productivity. In one of the medium, the solution was to remove a peptone and a CD component and in the other medium BD increased a single CD component.
Case Study #2 – Issue: Appropriate charge variant profile, but unacceptable cell growth and productivity.
In the second case study, BD was approached about another biosimilar, but this time the issue was that after initial screening the data showed appropriate charge variant profile in the control medium, but cell growth and productivity were not acceptable. Thus, the goal was to optimize the medium formulation to meet desired growth and productivity while maintaining the charge variant profile. To meet this goal they needed to achieve peak VCD of ≥ 5×106 cells per mL and production of ≥ 150% of control media. The control medium’s peak density was about 3×106 cells per mL.
Through careful study design, spent media analysis and analytical testing, the team at BD identified three hydrolysates for optimization from the original medium. Through a fractional factorial design, ten media were subjected to batch culture growth and productivity testing. Three media were found to meet the cell growth and production requirements. Charge variant profile analysis showed that all 3 media resulted in biosimilar IgG with the correct charge variant profile.
Case Study #3 – Develop a custom CD base medium and feed to increase productivity and maintain N-glycan profile.
In this case study, a customer, who had been using a commercially available CD medium, approached BD to develop a customized CD base medium and CD feed to enhance productivity while retaining existing quality parameters.
The goal was to develop a CD base medium and CD feed to enhance productivity at least 2 fold and maintain IgG N-glycan profile as compared to the commercial CD medium control.
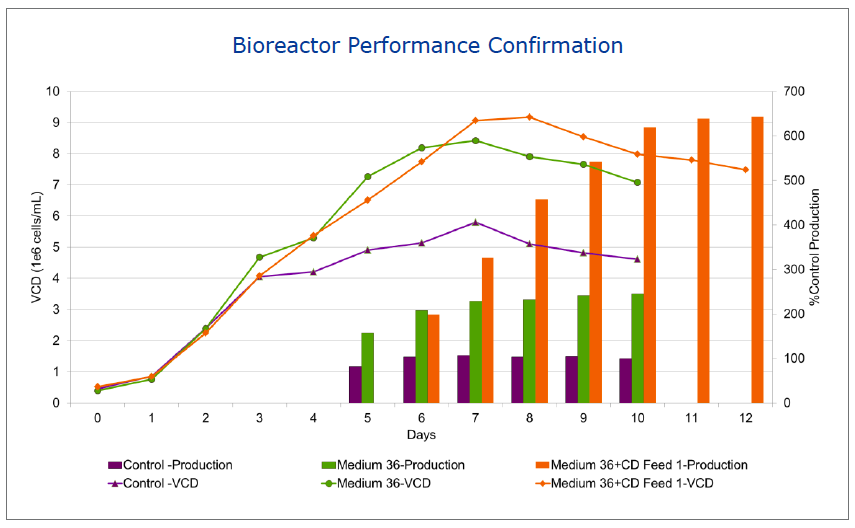
BD began a base medium and feed design project (Figure 1). To begin the project, the team conducted a library media screen of in-house CD media. From this screen, 8 media met productivity criteria, but none, at this point, met growth requirements. Several from the deep well plate screen were scaled up to shake flasks. Based on productivity, growth and viability studies in the shake flasks, two formulations progressed to microbioreactor studies using the ambr system. The team then compared batch culture with two CD feeds and based on these results, moved forward with one medium and feed combination. This medium and feed was moved into a bench top bioreactor for confirmation that the medium goals had been met. The bioreactor performance confirmed that the medium and feed exceeded the goals. Productivity was up 6 fold from control while successfully maintaining the N glycan profile (Figure 2).
Figure 1:
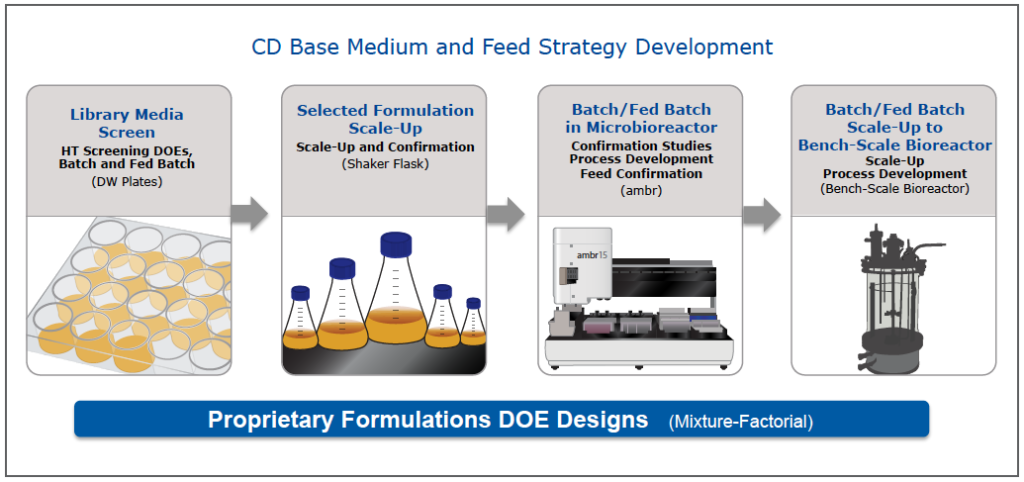
Figure 2:
Conclusions
Dr. Brooks concluded the webinar by pointing out that these case studies demonstrated how cell culture media optimization continues to play a critical role in both improving titers and providing a mechanism for modifying protein quality. Furthermore the supplements chosen to enhance media can have dramatic effects, both positively and negatively, on productivity, growth and protein quality. This highlights the importance of knowing what the drivers of the cell culture process are in order to identify appropriate supplements.
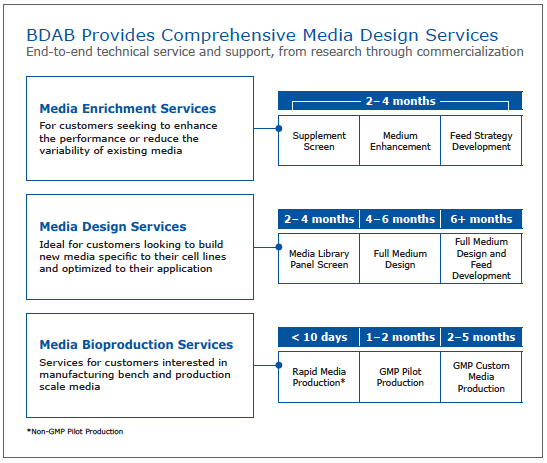
Lastly, Dr. Brooks discussed the types of services that are available through media design services and expected timelines. This provides a good reference for those interested in outsourcing development and optimization projects (Figure 3).
Figure 3: