A Complete System for Viral Vector and Recombinant Protein Production in HEK293 Cells
HEK293 cells are easy to grow in culture and commonly used for transfection to produce recombinant protein or gene products. Transfection of HEK293 cells is prevalent due to the fact that they are easy to transfect with high efficiency, and in the right culture environment, can achieve high levels of expression quickly. Two applications with widespread use of HEK293 cells are viral vector production for gene therapies and recombinant protein production.
Viral Vectors for Gene Therapy
The research behind Gene Therapy demonstrates that a correct gene copy can replace a missing or defective gene. However, since DNA cannot be directly inserted into cells, Gene Therapy requires a carrier or vector, most commonly inactivated viruses.
Transfection of HEK293 cells with vector and packaging plasmids is the most widely used method to produce retrovirus, lentivirus, and adeno-associated virus (AAV) viral vectors used in Gene Therapy. According to data published by the Journal of Gene Medicine, there are over 2,200 gene therapies in clinical trials, while the majority of these trials are still in Phase I/II, there are some treatments progressing toward commercialization. With the increase in Gene Therapy projects, the demand for efficient and scalable viral vector manufacturing is increasing, along with the need to provide an appropriate culture environment to enable efficient transfection of HEK293 cells.
Small to Large Scale Production of Recombinant Proteins
HEK293 cells have long been utilized for transient protein expression. And in recent years the use of HEK293 for large scale production of recombinant protein has become more prevalent, as it enables the quick and efficient production of milligram to gram-scale yield of recombinant proteins.
HEK293 Cell Culture and Transfection
In addition to the method of transfection, the cell culture environment is also important in enabling fast and highly-efficient transfection of HEK293 cells. In order to achieve high titer, be it protein, or viral vector, it is important to choose the appropriate cell culture media system. The media must not only support the growth of the HEK293 cells, but also facilitate the efficient transfection of the cells, and promote elevated titers.
To respond to the need for a simple and efficient media system for HEK293, Irvine Scientific recently launched BalanCD® HEK293 system. This media system is chemically-defined, animal component-free, and designed to support growth and transfection of HEK293 cell lines in suspension cultures, and supports a range of applications including production of viral vectors and recombinant protein production.
Highlights of the BalanCD HEK293 system include:
Improved Productivity of Viral Vectors
The BalanCD HEK293 System for viral vector production comprises BalanCD HEK293 medium and an Anti-clumping Supplement. The versatile BalanCD HEK293 medium enables scientists to use the same medium for transfection and rapid, scalable production of viral vectors. The Anti-clumping Supplement prevents cell aggregation when added to the culture medium; the supplement should be completely eliminated from the cell culture prior to transfection, and/or added at least 24 hours post-transfection, as it completely inhibits transfection.
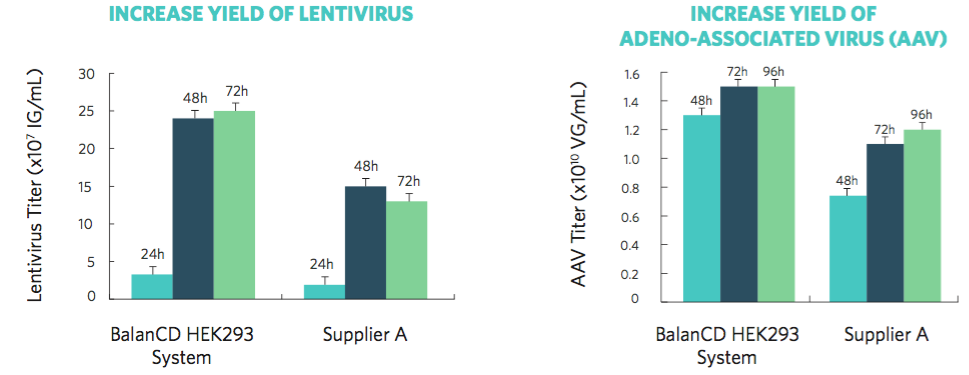
Data, provided by Irvine Scientific, show optimal production yields and higher virus titer achieved within 48 hours. When compared to other commercially available 293 media, cells cultured in BalanCD HEK293 medium showed higher productivity, with a 60% higher lentivirus titer and a 40% higher AAV titer (Figure 1).
Increased Recombinant Protein Production and Culture Longevity
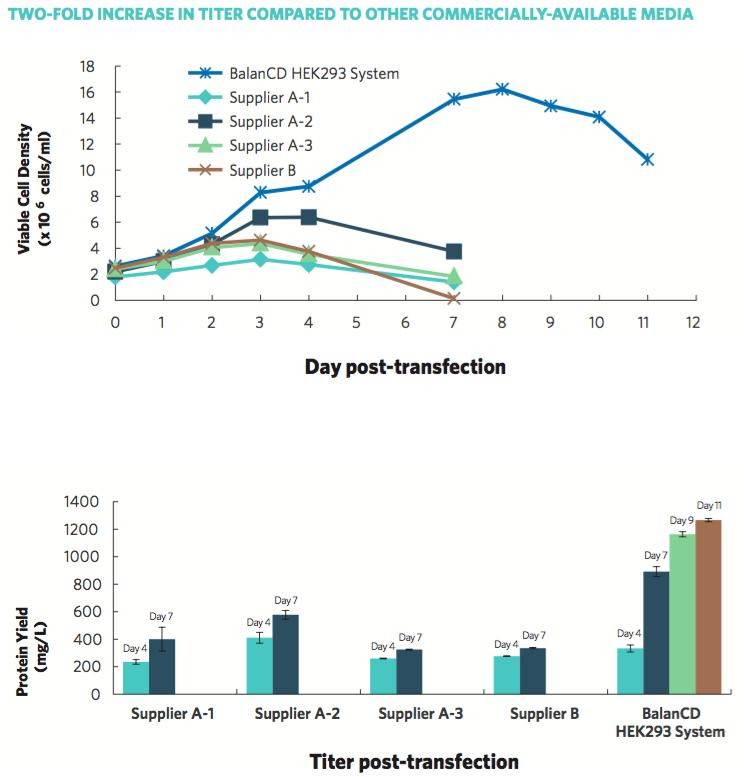
In order to meet the need for an efficient, high-yield protein production system, Irvine Scientific developed BalanCD HEK293 Feed as part of the BalanCD HEK293 System. BalanCD HEK293 Feed, when added to cell culture containing BalanCD HEK293 medium and Anti-clumping Supplement, enables higher cell growth of HEK293 cells, and significantly increases protein yield. When compared to other commercially-available media, HEK293 cells cultured in the BalanCD HEK293 System showed higher viable cell density, over a longer culture duration, and more than 2-fold protein yield, according to data provided by Irvine Scientific (Figure 2).
Versatility
A recent poster titled, “A Versatile and Scalable Media Platform Designed for Cell Based Applications Using Human Embryonic Kidney 293 Cells,” provides additional data on the use of BalanCD HEK293 for Gene Therapy applications.
For more information, please contact Irvine Scientific: Tel: +1 949 261 7800; Email: getinfo@irvinesci.com