
SCOUT® Technology Reduces Time to Market and Increases Chance of Success for Biopharmaceutical Products
Introduction
Only 1 out of each 50 biopharmaceutical new product candidates makes it through the research phase into clinical trial testing and subsequently to the market. This high attrition rate is predominantly in the early development phases and is attributed to (I) undesired pharmacokinetics profile (39%), (II) lack of efficacy (30%), (III) in vivo toxicity in preclinical model (11%), (IV) adverse effect in humans (10%), and (V) other reasons, of which most commonly commercial arguments based on cost of goods (10%). It is therefore imperative that technologies become available that allow significant de-risking of biopharmaceutical product trajectories in the early research and development phase. The importance of this has been recognized by the field with the introduction of the “Design of Experiments” (DoE) approach, identifying critical quality attributes and performance attributes like yield, glycosylation, potency, and consumable costs of a manufacturing process. Owing to the DoE approach, scientists now have a tool to strategize the development of a novel product candidate. That said, it is often found that due to the complexity of many novel molecules, the number of parameters that need to be tested still requires vast numbers of experiments which are time consuming and costly. Batavia Biosciences has developed SCOUT®, a platform that integrates scaled down, high throughput cell culture systems with high throughput purification and analytical capabilities. This cool tool allows for the rapid and cost effective generation of vast data sets and has proven to help you to de-risk your program in the early phases of development and significantly improves the success rate.
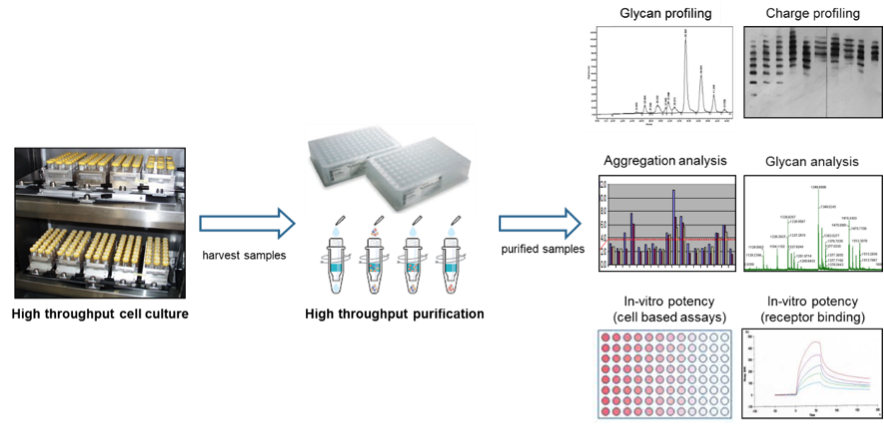
SCOUT® technology
The SCOUT® platform represents a qualified, scaled-down high throughput system for optimizing upstream and downstream processes for all major classes of biopharmaceuticals be it proteins, antibodies, viral vaccines, or viral vectors for Gene Therapy protocols. In the upstream development SCOUT® is frequently used for, but not limited to, yield and growth optimization projects and has proven its worth with documented increases in yield of at least 5-fold. The upstream process is typically performed at 2-20 mL scale and allows hundreds of cultures to be tested in parallel. Results obtained with SCOUT® have been successfully scaled up to 1000 L bioreactor volume hence demonstrating scalability of the obtained results. In downstream development SCOUT® is used to process high numbers of samples using scaled-down purification technology ranging from 50 μL (96 well plates) to 2 mL tubes (customized spin filters and spin traps). With this capability, your downstream development can already be initiated when upstream development is still in progress, providing only limited amounts of material. The high throughput, SCOUT® technology is able to provide sufficient purified material to generate robust, representable analytical data, including glycan profiling, aggregation analysis, glycan analysis, and in vitro potency testing. See figure 1 for a graphic representation of SCOUT®.
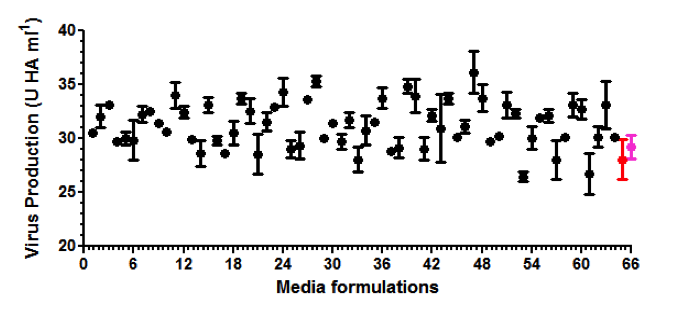
After demonstrating that the SCOUT® upstream and downstream technology mimicked data from a commercial influenza vaccine manufacturing process (1000 L; HA purification by centrifugation; three influenza virus strains), the technology was used to screen for hundreds of new production media with the aim to increase the fraction of soluble HA for each strain. In total, the experiment was performed in one run with 150 different production media, 3 different influenza strains, 5 samples per medium, thus 2250 conditions were tested. The entire experiment was executed within three weeks’ time and fully occupied only two skilled technicians.
The example with influenza virus described above was based on the MDCK platform where the cells were growing on micro-carriers. Batavia has mastered the growth of a wide range of mammalian cell lines (CHO, HEK293, Vero, and MDCK) as well as diverse microbial species including E.coli for optimization of protein production for growth in its SCOUT® system.
In conclusion, SCOUT® provides a powerful cost effective cool tool in biopharmaceutical product development and is employed by Batavia Biosciences hand-in hand with the DoE approach and Quality by Design principles as outlined in the FDA guidance on Pharmaceutical Development (Q8).
Would you like to learn more about Batavia Biosciences? Please visit our website at www.bataviabiosciences.com.