
COVID-19 Therapeutics in Clinical Trials: The Antivirals
As the SARS-CoV-2 pandemic spreads around the world in early 2020, scientists, doctors, and clinicians are rushing to find effective therapies for COVID-19 disease. Approved and investigational medications with a spectrum of activities are being examined as to whether they can be repurposed for COVID-19. Due to the magnitude of the effort by the medical community, it is difficult to keep track of the numerous therapeutic approaches.
We scanned clinicaltrials.gov and found roughly 50 medications, world-wide, that have been in trial, currently are in trial, or are soliciting applicants for study. The medications include a broad array of activities which include antivirals, antiviral cell mediators, immunomodulators, and others. They are being evaluated singly, or in combinations, and usually across multiple clinical sites.
In this report, we will review a subset: antivirals and antiviral cell mediators, with a highlight on their activities.
We found that antivirals being examined for SARS-CoV-2 can be separated into 2 broad classes as to whether they interact directly with a viral expressed component (antivirals) or components of the cell (antiviral cell mediators) (Table).
| Therapeutic | Therapeutic Class | Mechanistic Category | Company | Notes |
|---|---|---|---|---|
| Direct Antivirals | ||||
| Remdesivir | Antiviral | Antiviral | Gilead | FDA approved emergency use; broad sprectrum; RNA chain-terminator |
| Favipiravir (Avigan) | Antiviral | Antiviral | Toyama Chemical/Fujifilm | Approved JN, CN; broad spectrum; RNA chain terminator, mutagenic |
| Emtricitabine/tenofovir (Truvada) | Antiviral | Antiviral | Gilead | HIV medication; DNA chain terminator |
| Danoprevir+Ritonavir | Antiviral | Antiviral | Array BioPharma/Roche | Investigational; HCV NS3/4A protease inhibitor |
| Darunavir/Cobicistat (Prescobix) | Antiviral | Antiviral | JNJ/Janssen | Approved HIV protease inhibitor. Preliminary study: no efficacy for COVID-19 |
| Lopinavir/Ritonavir (Kaletra) | Antiviral | Antiviral | Abbvie | Approved HIV protease inhibitor; |
| Oseltamivir (Tamiflu) | Antiviral | Antiviral | Genentech | Approved sialiac acid homolog NA inhibitor; influenza antiviral |
| Umifenovir (Arbidol) | Antiviral | Antiviral | Pharmstandard | OTC in Russia for influenza; affect viral entry |
| Cellular Effects | ||||
| Hydroxychloroquine | Antiinfective | Antiviral, cell mediated | Multiple | Approved antimalarial; multiple cellular effects; lower endosomal pH and other effects |
| Atovaquone (Meprin) | Antiinfective | Antiviral, cell mediated | GSK | Approved antimalarial, pneuocystic, toxoplasmd; interacts with electron transport |
| Nitazoxanide | Antiinfective | Antiviral, cell mediated | Romark Pharmaceuticals | Approved anticryptospordium, gardia; multiple mechanisms; has antiviral properties |
| Verapamil | Antiarrhythmic | Antiviral, cell mediated | Multiple | Approved antiarrhythmic; calcium channel blocker; may inhibit virus entry |
| Amiodarone | Antiarrhythmic | Antiviral, cell mediated | Multiple | Approved antiarrhythmic; calcium channel blocker; may inhibit virus entry |
| DAS181 | Antiviral agent | Antiviral, cell mediated | Ansun Biopharma | Investigational influenza entry inhibitor; sialidase fusion protein cleaves viral receptors |
| Pegylated Interferon lambda-1a | Antiviral | Antiviral, cell mediated | Eiger Biopharmaceuticals | Investigational antiviral previously investigated for HCV, HBV infection |
| Imatinib (Gleevec) | Antineoplastic Agent | Antiviral, cell mediated | Novartis/Multiple | Approved inhibitor of Abl2 kinase activity; viral fusion inhibitor |
Antivirals
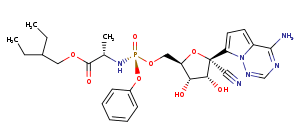
Originally developed to target Ebola, remdesivir is a prodrug adenosine analog delivered by I.V. that results in the chain-termination of viral RNA. It has shown activity against a wide range of RNA viruses including SARS-CoV-1, MERS-1 CoV, and other CoVs. Cell culture studies in Vero E6 cells show potent activity against SARS-CoV-2 with an EC50 in the low µM range (1.76 µM, 23.15 µM ) when added at the time of infection. Gilead’s Remdesivir received emergency use authorization on May 1 based on data from a NIAID and the SIMPLE trial. Studies show that remdesivir shortens hospitalization time from 15 days to 11 and that a 5-day regimen is similarly as effective as a 10-day regimen. Although no conclusions have yet been drawn on increased survivability, these results are encouraging.
Animal studies using SAR-CoV-2 in rhesus macaques, showed significant viral titer reduction of ~ 2 log10 in the lower respiratory tract, but not the upper tract. Lack of progression of disease was noted as well. Studies using SARS-CoV-1 in a transgenic mouse model also resulted in lower viral titers and greater pulmonary function if remdesivir was administered prior to or within one day of infection. Overall, current cell, animal and clinical data to date looks extremely promising for remdesivir.
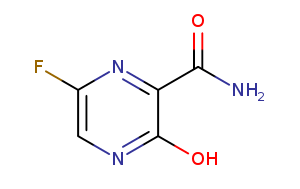
Favipiravir is an oral flu medication marketed as Avigan in Japan. It is a nitrogenous base prodrug. The active form interacts with the viral RNA polymerase to result in chain termination, although lethal transition and inversion of viral RNA can also occur.
Favipiravir has broad activity against RNA viruses including poliovirus, rhinovirus and Ebola. It has activity across numerous influenza strains including oseltamivir (Tamiflu) resistant strains with an EC50 in the low µM range. The EC50 against SARS-CoV-2 has been reported to be 61.88 µM in Vero E6 cells.
A small controlled study of 35 patients examining the effect of α-interferon + favipiravir vs α-interferon + lopinavir/ritonavir showed improved chest imaging and faster viral clearance in the favipiravir arm. However, the limitation of this study is that it was not randomized double-blinded and placebo-controlled.
Favipiravir was approved in Japan for treating influenza strains unresponsive to current antivirals. However, animal experiments showed the potential for teratogenic and embryotoxic effects and current production is limited to emergency stockpiles for novel or emerging influenza outbreaks. In March 2020 the drug was approved in China in for the treatment of influenza.
Repurposed HIV and HCV inhibitors
Emtricitabine/tenofovir (TDF) (Truvada) has proven effective for both the prevention of HIV infection and for the suppression of HIV replication in individuals (when combined with a third antiviral). These compounds are nucleoside and nucleotide analogs, which function via chain-termination of HIV viral DNA.
However, HIV utilizes an RNA-dependent DNA polymerase and CoVs utilize an RNA-dependent RNA polymerase and there is lack of data of their effectiveness with CoVs in vitro. A spokesman for Gilead Sciences states the company doesn’t anticipate favorable results because components of Truvada were shown to be ineffective against other viral diseases.
The coronaviral proteases, papain-like protease (PLpro) and 3C-like protease (3CLpro), are attractive antiviral drug targets because they are essential for viral polyprotein processing. There have been uncontrolled reports that some HIV and hepatitis C virus protease inhibitors improved the clinical outcome of patients. There are a number of trials evaluating repurposed protease inhibitors.
Darunavir is an effective tight binding competitive inhibitor of the aspartic protease of HIV with an EC50 of 1-8.5 nM. Cobicistat is inactive against HIV and serves boost the level of darunavir via inhibition of the liver’s cytochrome P450 3A system. To date there have been only anecdotal reports of the effectiveness of darunavir for COVID-19.
In that regard, J&J and Janssen have issued a statement that it has obtained no clinical nor pharmacological data to support darunavir’s use for treatment of COVID-19. This includes their own in vitro data that shows that viral inhibition occurs only at concentrations that are above those achieved in patients.
HIV protease inhibitor lopinavir and its booster ritonavir (Kaletra) appeared to be clinically effective in some studies (1,2) with COVID-19 patients, and in a small study it appeared to prevent progression of MERS. However, in these studies Lopinavir/Ritonavir was combined with ribavirin. A recent controlled trial of 200 COVID-19 patients found no clinical improvement. Moreover, in vitro data in Vero E6 cells has reported an EC50 of 26.1 µM which is above the concentration achievable in patients with twice daily dosing.
Danoprevir is a potent peptidomimetic competitive inhibitor of NS3/4A protease of Hepatitis C virus (HCV) with a reported EC50 activity in the low nM range in huh-7 cells harboring a HCV replicon. A small study of 11 COVID-19 patients, which was released as a non-peer reviewed preprint, claimed reduced hospitalization time with the combination of danoprevir and the booster ritonavir. Currently, there appears to be a lack of in vitro and clinical data to support its use.
Oseltamivir (Tamiflu) is a neuraminidase inhibitor that is approved for influenza. Oseltamivir has no demonstrated in vitro activity against SARS-CoV-2, and the virus lacks neuraminidase activity.
Umifenovir (Arbidol) is a broad spectrum anti-viral that is approved in Russia for influenza. Its exact mechanism is not completely understood, but it appears to block viral entry or fusion with the cell. Drug-resistant influenza virus have been isolated and the mutations primarily map to the viral surface HA protein. This suggests that the drug may interact with viral surface glycoproteins. Umifenovir also has additional activities and has been reported to stimulate immune responses.
A limited nonrandomized study of 66 patients with COVID-19 showed a 9-day regimen of umifenovir was associated with higher discharge rates and lower mortality. However, another small study in China with 120 patents with mild symptoms showed that umifenovir was inferior to favipiravir for the reduction of fever and cough.
Cell mediated antivirals
Hydroxychloroquine (HC) is considered a less toxic derivative of chloroquine (CQ). Both are currently in use for malaria. In addition to its antiparasitic effect, these drugs have immunomodulatory effects and are used for the treatment of lupus, rheumatoid arthritis, and porphyria cutanea tarda.
HC and CQ have been reported to have broad in vitro antiviral activity against diverse viruses including poliovirus, HIV, hepatitis A and influenza, although observations on whether these activities translate in animals has been both limited and conflicted. HC and CQ have been shown to inhibit SARS-CoV-2 in vitro using Vero E6 cells with an EC50 in the low µM range (1, 2, 3) with some reports suggesting that HC may be more potent (1,2).
Since these drugs have numerous effects on cellular activity, the exact mechanism of antiviral activity is not clear and may involve multiple activities. Both drugs are known to raise endosomal pH, which may influence viral entry or glycosylation. Other proposed mechanisms include altered cell receptor glycosylation, inhibition of viral protein processing, and increased levels of intracellular zinc.
The numerous cellular effects of these therapeutics may explain the diversity of the therapeutic effects as well as the, sometimes serious, adverse effects of these drugs. Clinical studies of their use for COVID-19 have been both limited and controversial. Greater evaluation is needed to understand whether there is a clinical benefit.
Atovaquone (Meprin) is used in the treatment of malaria, pneumocystis pneumonia , toxoplasmosis, and babesia. Its mechanism is not fully understood. As an analog of inner mitochondrial protein ubiquinone (coenzyme Q), its activity is thought to be related to the lowering of nucleotide pools, including ATP.
Although it has been documented that atovaquone inhibits chikungunya and zika virus in vitro in low µM concentrations, there is a lack of study to demonstrate in vitro activity against coronavirus. However, computer modeling has suggested an interesting potential interaction with SARS-CoV-2 protease.
Nitazoxanide is a broad-spectrum antiparasitic and antiheminth and is used for the treatment of infection by Cryptosporidium, Giardia, and others. Its antiprotozoal activity is thought to be related to anaerobic energy metabolism, although other cellular effects have been noted.
Nitazoxanide has broad spectrum antiviral activity in vitro against influenza and other virus including HBV, HCV, HIV, rotavirus, norovirus, and others. Nitazoxanide is currently being developed for treatment of influenza. A recent Phase 2b/3 trial for influenza found that a 5-day regime reduced the duration of clinical symptoms and reduced viral sheading vs. placebo.
In vitro data of SARS-CoV-2 in Vero E6 cells found an EC50 of 2.12 µM. Although the activity profile and in vitro data for nitazoxanide appears highly promising, more animal and clinical evaluation is needed to support its potential for COVID-19.
Two interesting medications are verapamil and amiodarone which are well-known antiarrhythmic drugs used for treatment of ventricular and supraventricular arrhythmias. They block voltage-dependent calcium channels to decrease impulse conduction through the AV node. They also have some affinity for potassium channels. While both drugs block Ca and K channels each of them has other unique interactions and activities. Both of these drugs have been reported to have anti-viral activity based on in vitro data.
Verapamil has been reported to inhibit human rhinovirus production and release with the majority of the effects on release. The activity was reportedly not linked to the blockage of Ca+2 influx. Verapamil also inhibits influenza. The mechanism of influenza inhibition appears to involve multiple, diverse effects that result in strongly reduced viral production. These include inhibition of the viral polymerase, reduced NF-κB activation, and decreased viral protein production. There is currently no clinical data on the efficacy of Verapamil for COVID-19.
Amiodarone has been shown to block inhibit SARS-CoV-1 infection in vitro. The mechanism is not fully understood but there is data that the drug affects viral replication after early transit of the virus through the endosome.
DAS181 is a recombinant sialidase protein that cleaves sialic acid on the surface of epithelial cells lining the human respiratory tract. It is an inhaled therapeutic. Many viruses interact with sialoside residues as part of the cell binding process and treatment with DAS181 is currently in Phase IIb/3 trials for treatment of influenza. The recognition of sialic acid receptors of other human coronaviruses has been determined on the structural level (1, 2) and there is some analysis that suggests a similar recognition for SARS-CoV-2.
Interferon Lambda-1a is an investigational interferon with reported in vitro activity similar to interferon alpha-1 when tested using hepatitis C virus in hepatocytes. Phase IIb and Phase III studies with HCV patients with comparative treatment regimens showed that pegylated interferon lambda-1a produced a similar positive clinical effect compared to pegylated interferon a-1 with a reduction of unwanted side effects. The two interferons differ in the cellular receptor they use that results different cell tropisms. This difference in cell tropism might explain the improved side effects. The effects of pegylated interferon lambda-1a on SARS-CoV-2 in vitro has not yet been published nor have there been previous clinical studies for COVID-19 therapy.
Imatinib (Gleevec) is an oral medication for cancer that is used for some types of leukemia, gastrointestinal stromal tumors, hypereosinophilic syndrome, systemic mastocytosis, and myelodysplastic syndrome. Imatinib inhibits tyrosine kinases and its activity to inhibit Abelson tyrosine-protein kinases (Abl) is thought to result in its antiviral activity.
Abl2 activity is thought to be a required step to initiate viral fusion with the cell endosomal compartments. Imatinib inhibits SARS-CoV-1 and MERS-CoV infection in Vero E6 cells with EC50 values of 9.82 and 17.69 µM. A 25 µM concentration of imatinib can inhibit viral production 1,000-fold when added within 4 hours of infection. If added within 5 hours of infection no inhibition was observed. These observations support that the hypothesis that imatinib acts as an inhibitor of coronavirus viral entry.
Summary and Perspectives
Our survey shows a wide variety of investigational and approved repurposed antivirals and cell modulators are being investigated for COVID-19. The activities of these medications are diverse. The cell modulators include activities as that range from antiparasitic, to antiarrhymic, to antineoplastic.
We also note that antivirals that are rationally designed to be specific for SARS-CoV-2 have yet to appear to the clinical scene. Rational design is likely to produce tighter interaction with targets and increased specificity. This in turn, can result in lowered dosing and reduced side effects.
While none of these medications, at this time, appears to completely inhibit viral production as a monotherapy, there is continued optimism and that a combination of medication approach may deliver robust therapeutic benefit. The combinational approach has proven successful with HIV and can lower HIV levels in patients to undetectable levels. Indeed, there is at least one report where additive or synergistic effect was observed between two antivirals for SARS-CoV-2 in vitro; the combination of remdesivir and the antiprotozoal emetine.