Defined, Animal-free Virus Production Media
In our previous blog “The Evolution of Vaccine Manufacturing – Past, Current, and Future Trends,” we discussed the importance of continued innovation in vaccine manufacturing. These innovations are critical to reducing manufacturing costs and improving safety profiles. As discussed in the blog, several vaccines are currently being manufactured with a cell culture media formulation that contains animal derived components. The optimization of cell culture media and the removal of these animal products is one area where improvements can be made in vaccine manufacturing.
Another area where vaccine manufacturing is evolving is in the use of newer vessels, including bioreactors. Bioreactors are currently being used in vaccine manufacturing and their use will likely continue to expand. Bioreactors can provide cells the optimum environment, which can lead to an increase in productivity and reduced overall cost. While many vaccines are still manufactured using static culture or roller bottles, several studies have shown that manufacturing can be greatly improved by employing microcarriers and stainless steel or single-use stirred tank bioreactors.
Microcarriers enable more cells per milliliter of culture by expanding the surface area for cell proliferation. More cells in culture increases overall vaccine titer (yield). With an increase in the use of stirred-tank or single-use bioreactors, microcarrier innovations have been necessary to allow adherent cells to be cultured in these conditions. In addition, the use of microcarriers allow manufacturers to increase the number of cells that can be cultured in one tank enabling more efficient large-scale production and permitting the use of greater than 1,000 liter bioreactors.
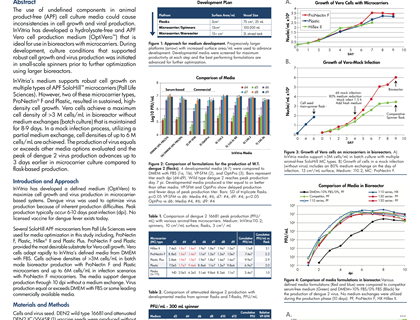
Last week at the World Vaccine Congress in Washington DC, Dr. Steve Pettit, Director, Cell Culture Product Development, InVitria, presented a poster that addresses both of these areas of innovation. The poster titled “Robust, Defined, Animal-Free Virus Production Medium Optimized for Microcarrier Culture,” was jointly authored by InVitria, PALL Life Sciences, US Centers for Disease Control and Utah State University. It highlighted InVitria’s development of a defined, hydrolysate-free and animal product-free virus production media for vero cells (OptiVero). The medium was designed specifically for use in bioreactors employing microcarrier technology. In the development of the product, InVitria used dengue virus because of the inherent production difficulties associated with this virus. They employed SoloHill APF microcarriers from PALL Life Sciences for media optimization studies. While several of the microcarriers tested provided good results, two in particular supported sustained, high-density cell growth, ProNectin F ® and Plastic.
“With collaborators, we have shown success achieving high cell density and viral titer with this novel medium formulation using dengue vaccine as a prototype,” said Dr. Steve Pettit, Director, Cell Culture Product Development. He continued, “We are working to expand on this work to help other vaccine producers eliminate the risk of using animal serum components”.
For more information, please view a copy of the poster – “Robust, Defined, Animal-Free Virus Production Medium Optimized for Microcarrier Culture,”