
A Demonstration of Effective Scalability of Suspension Cells for the Production of rAAV Viral Vectors using the Allegro™ STR bioreactor System
Reliable and scalable viral vector manufacturing platforms are needed to accommodate the increased demand as more gene therapy treatments are brought to market. These transformative medicines can be used to replace a defective gene with a functional copy, silence a defective gene or even be introduced to directly edit genes in vivo. Not only is the diversity of target indications growing, but the size of the patient population is as well, necessitating better upstream technologies to meet the increased viral vector demand that drive these therapies.
Viral vectors based on recombinant adeno-associated viruses (rAAV) are commonly used for gene therapy strategies due to their diverse tropism, minimal immunogenicity, and efficient and persistent gene transfer. Transient transfection of adherent HEK293 cells is a common production vehicle, however many developers have adapted these cell lines to grow in suspension culture, to take advantage of bioreactor technologies that allow for scalable production and higher productivity. Triple transfection with plasmid DNA encoding viral proteins, induces rAAV vector production within the host cells, which continue to expand in culture. The viral vector product is subsequently harvested from the cells and formulated for use, either directly as a gene therapy or to create ex vivo treatments using patient cells, such as in CAR-T cell therapies.
Improving manufacturing scalability and productivity of viral vectors is a goal for many companies invested in gene therapies. Abeona Therapeutics is a gene therapy company developing novel gene replacement therapies for rare inherited diseases. In an effort to optimize production processes for their rAAV-based gene therapy product, Abeona Therapeutics evaluated the Pall Allegro™ STR single use family of bioreactors as a potential solution to the scalability problem. Process scalability at the 50 L and 500 L working volume was evaluated based on key process parameters such as cell growth, viability, metabolic profile, and vector production. The comparability study entitled Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector was recently published in Cell & Gene Therapy Insights (September 2021). A brief summary of the findings is outlined here.
Suspension-adapted AAV293 cells from Abeona’s master cell bank were used to establish a seed train to inoculate an Allegro STR 50 bioreactor at a density of 0.2 x 106 cells/mL in a 20 L volume. This culture was expanded to a final working volume of 50 L two days later to serve as the N-1 bioreactor. The cell culture bolus from the N-1 bioreactor was used to prepare a uniform inoculation pool of 275 L in the STR 500 from which 25 L was transferred to a parallel control STR 50, representing 50% volumetric capacity for each bioreactor. After 24 hours, the cultures in the test and control vessels were then expanded to the full working volume, 475L and 47.5L, respectively, and maintained until the target transfection density of ∼1.0 x 106 cells/mL was achieved. The process parameter scale-up strategy utilized constant power per unit volume (P/V), while maintaining scalable flow (vvm) for both sparge rate and overlay. The viable cell density (VCD) and viability of the cultures were monitored daily along with glucose and lactate analyses. pH and dissolved oxygen (DO) data was also collected throughout the culture period and analyzed. Cell growth and viability between both the STR 50 and STR 500 was found to be nearly identical throughout the culture period, while some minor differences in metabolite profiles were observed post-transfection. The pH at both scales trended together and both bioreactors maintained DO at the 50% setpoint through normalized O2 sparge rate control.
PEIpro™-mediated transfection of plasmid DNA was performed according to Abeona’s transfection protocol for each bioreactor separately followed by DENARASE® treatment 24 hours later. Cells were harvested post-transfection when viability fell below 20%. Samples from the STR 50 and STR 500 bioreactors were collected starting 7 days post-transfection until harvest (day 15), to assess vector production capacity using digital droplet PCR. rAAV titer increased throughout the culture period with maximum titers observed at harvest (Figure 1). The final titer was found to be higher in the 50 L vessel compared to the 500 L vessel (4.8 x 1010 gc/mL and 4.3 x 1010 gc/mL respectively).
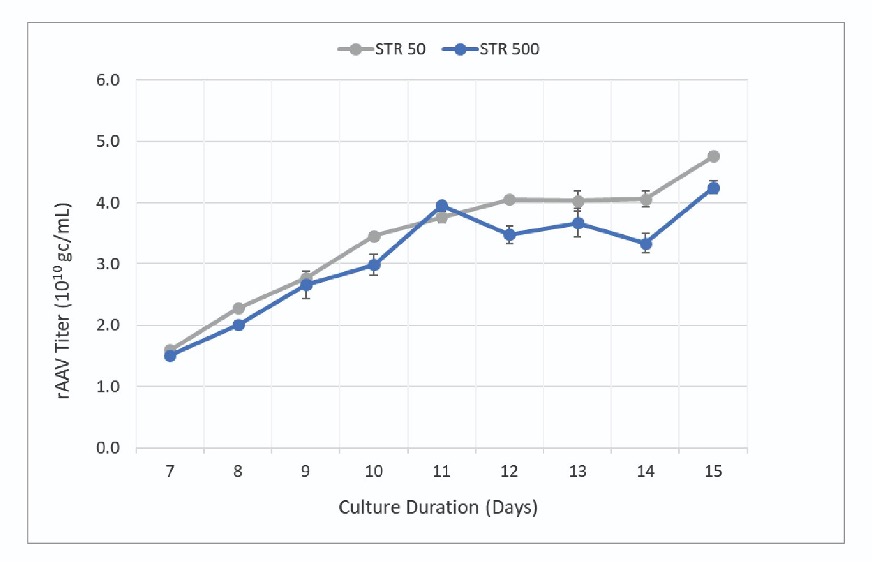
The data presented by the authors effectively demonstrate the scalability of Abeona’s transfection-based rAAV production process between the Allegro STR 50 and STR 500 bioreactors. Analysis of key process parameters showed similar growth kinetics, viability and vector titers throughout the evaluation period. While slightly higher titers were observed in the STR 50, the productivity between the two scales was within ∼10% indicating a scalable process.
The ability to quickly and effectively scale-up viral vector production is necessary to address the increased demand across the industry as more gene therapies enter late-stage clinical trials towards market approval. The ability to leverage scalable production platforms such as the Allegro STR suite of bioreactors makes process development and subsequent scale-up more streamlined offering time and cost savings that ultimately benefits high-need patients.
To download a copy of the publication, please see Scalability comparison between 50 and 500 liter stirred tank bioreactor for production of rAAV viral vector