
Demystifying Cord Blood: Answers to Common Questions from Cell Therapy Developers
Allogeneic cell and gene therapy developers considering cord blood as the source material for their therapy recognize the advantages it holds—from its naïve state to being cryopreserved and readily available.
However, developers often have questions for our team at Be The Match BioTherapies. This article covers common questions about factors such as licensure, characterization, and efficient access to the domestic cord blood supply.
What is the difference between licensed and unlicensed cord blood units?
This is one of the first questions we get from cell and gene therapy developers.
Eight cord blood banks in the United States have received a biologics license application (BLA) approval from the Food and Drug  Administration (FDA) to distribute cord blood units (CBUs) as a graft source for allogeneic hematopoietic stem cell transplantation (HSCT). This means the FDA reviewed and accepted the cord blood bank’s operations for product manufacture.
Administration (FDA) to distribute cord blood units (CBUs) as a graft source for allogeneic hematopoietic stem cell transplantation (HSCT). This means the FDA reviewed and accepted the cord blood bank’s operations for product manufacture.
Licensed CBUs meet all the requirements under the BLA. Some CBUs may not meet every BLA requirement and, therefore, are considered unlicensed. The BLA is specific to allogeneic HSCT.
Cord blood banks also have research-use-only units that are manufactured and characterized similarly to licensed and unlicensed units but do not meet the eligibility and safety requirements for use in humans for reasons such as a donor’s medical history. These units are useful for validating, qualifying, and investigating internal procedures and processes or engineering runs.
Are unlicensed cord blood units inferior to licensed units?
An unlicensed CBU is not considered inferior to a licensed unit. Both are manufactured as clinical grade cellular products and meet donor eligibility and safety requirements for use in humans. Physicians use licensed and unlicensed CBUs in HSCT.
There are two main reasons a unit from a licensed cord blood bank is unlicensed. The first is timing. A cord blood unit cannot be licensed if it was processed and stored before a bank received its BLA approval. A cord blood bank may have used the same processes and equipment, but the BLA is not retrospective.
The second reason is the CBU collection or processing of the cord deviated from the specifications required in the BLA, such as a missing sample. Unlicensed units due to small process deviations are often still available for clinical use or research.
Unlicensed units are always released under IND. Because a cord blood bank’s BLA is for allogeneic HSCT, even units banked post-licensure and meeting criteria for classification as a licensed unit for transplant are released under IND for off-label applications, such as for use in a cell or gene therapy.
There are more unlicensed domestic cord blood units listed on the Be The Match Registry than licensed units.
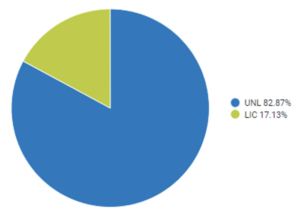
Cell therapy companies that are open to obtaining both licensed and unlicensed units will have a better likelihood of having a larger supply of cord blood units available for use. Biological Licensure is just one of a handful of ways public cord blood banks document and implement quality standards. Accreditation by AABB or NetCord FACT are also measures of quality which may provide quality assurance outside of licensure.
Which attributes are available for cryopreserved cord blood units?
The information available for the unit could be more important than a CBU’s licensed versus unlicensed status. Through Be The Match BioTherapies and our Cord Blood Bank Alliance, allogeneic cell and gene therapy developers have access to characterization information such as viral testing, cell viability characteristics like CD34+ counts and total nucleated cell (TNC) counts, HLA type, ABO/Rh, and infectious disease markers.
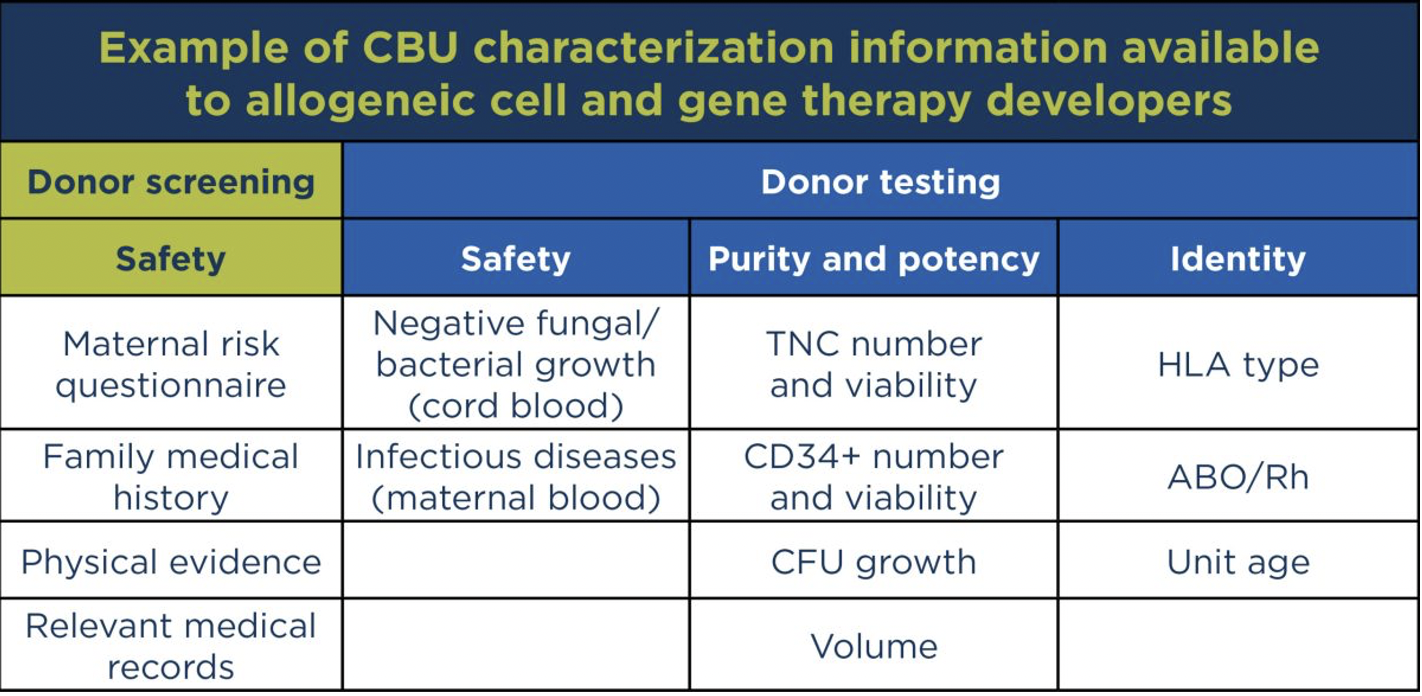
The data points available today are those needed for HSCT. However, in our experience, cell therapy developers are often looking for very similar attributes.
What is the most efficient way to access the U.S. cord blood inventory?
While a cell therapy developer could contact individual banks, Be The Match BioTherapies developed the Cord Blood Bank Alliance to simplify cord blood sourcing.
All cord blood banks that are part of the Alliance are existing members of the National Marrow Donor Program (NMDP)/Be The Match network. Be The Match BioTherapies is part of the NMDP/Be The Match. The banks have a history of providing high-quality cord blood grafts for HSCT and are motivated to propel the cell and gene therapy industry forward.
These domestic banks contract with Be The Match BioTherapies to supply CBUs for cell therapy development and commercialization. The members also meet regularly to share knowledge, evaluate cord blood banking practices, and consider alternative banking and attribute models to support cell and gene therapy applications.
Be The Match BioTherapies provides cell and gene therapy developers with a central point for searching and ordering cord blood units. Our experienced logistics team supports cord blood unit sourcing for cell therapy developers. For those who need support determining the best CBUs for their therapy development goals, our Bioinformatics team has data and analytical expertise to help define their selection parameters.
In addition, cell therapy developers can tap into the knowledge of Alliance cord blood banks. For example, banks have experience freezing and thawing units and have a strong understanding of all cell types in CBUs, not just CD34+ cells.
With the wealth of expertise available through Be The Match BioTherapies and the Cord Blood Bank Alliance, developers can get the answers they need to unlock the potential of cord blood for their allogeneic cell and gene therapy.
For more information, visit BeTheMatchBioTherapies.com.
 About the Author
About the Author
Christina Melief, PhD, Senior Scientific Advisor for Customer Ready Products at the National Marrow Donor Program/Be The Match.
Christina Melief, PhD, is the Senior Scientific Advisor for Customer Ready Products and a founding member of the Cord Blood Alliance at the National Marrow Donor Program/Be The Match. Dr. Melief worked previously in biotherapies supporting cellular material sourcing at a blood bank in the Pacific Northwest and as technical consultant for a global cell therapy company. She holds a PhD in genetics from Stony Brook University in Genetics. Since recently joining Be the Match, she devotes her time to advancing transplant and cord blood derived therapies through the Cord Blood Alliance, Cord Blood Advisory Group, and World Marrow Donor Association.