Developing Ebola Vaccine and Securing Your Cold Chain Logistics Plan
A guest blog by Dan H. O’Donnell, Associate Director of Cell Therapy Logistics, Fisher BioServices
Recent reports issued by the Centers for Disease Control and Prevention (CDC) show that efforts to contain the Ebola outbreak are yielding results. And even as we applaud the news, and also understand the need to continue the current efforts at an intense level, our collective attention is turning to the next step: developing a vaccine to prevent another outbreak.
Efforts to control transmission of the virus are working, and the number of new cases is falling rapidly in certain areas. The success of these efforts serve to highlight ongoing issues in halting transmission of the virus, as outbreaks are now appearing in more rural, hard-to-reach areas. The delivery of needed resources—people and supplies—where they are needed is increasingly hindered by logistical challenges as the virus moves into more and more remote locations.
Those who are in the front lines of vaccine development will soon face the same—or greater—logistical challenges as those currently working to contain transmission of the virus in rural villages.
The two vaccine candidates available, developed over the past decade in response to potential bioterrorism, are both being fast-tracked into clinical trials, and trials are already enrolling healthy adult volunteers in urban medical centers in the US, UK, Germany, and West Africa.
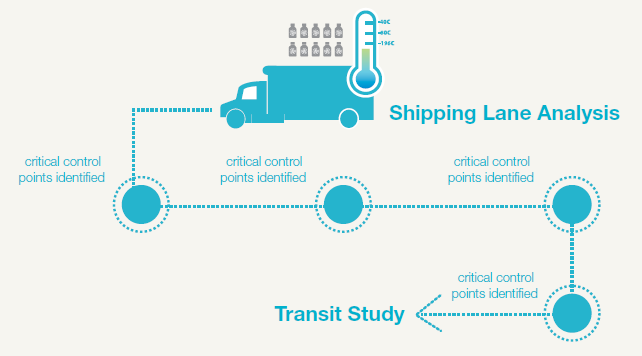
Distributing the vaccine to clinical trial sites presents a number of challenges, primarily that the vaccine is currently maintained at an ultralow temperature—that is, the vaccine is not merely frozen, but frozen to -80°C. To maintain correct temperature, the vials have to be shipped with dry ice, which sublimates directly into carbon dioxide gas and is therein considered a dangerous substance from a shipping point of view (I have previously discussed “testing samples shipped on dry ice” here). The shipping cartons must also carry a temperature monitor, so the receiving clinical site can be sure that the doses were maintained at the correct temperature and have not lost potency. Failure to collect temperature data, and verify that all doses administered to the clinical trial participants were handled appropriately, will endanger the clinical trial protocol and delay commercial approval.
Fisher BioServices supports clinical trials of these biological therapeutics, and knows the challenges involved, even in supporting clinical trial in urban centers with reliable infrastructure (see our case study on our cold chain transport project to Uganda). However, the challenge of delivering doses for clinical trials pales compared to the logistics of delivering frozen vials to the rural residents of Western Africa, who are most in need of the vaccine.
This logistical challenge may be the most pressing issue ahead. Scale-up of manufacturing is most often the critical issue with new therapeutics and preventives. In this case, scale-up of vaccine manufacturing is proceeding ahead of the clinical trials—an unprecedented move!
If the many organizations collaborating on the development of the two vaccines can eventually establish that the vaccine remains stable and maintains therapeutic potency at -20°C or even under refrigeration, the logistics will be significantly simplified. The doses can be shipped with frozen gel packs or other arrangements instead of dry ice; once the doses reach the destination country, keeping it refrigerated or frozen at the more typical -20°C will be much easier, as the equipment is far more widely available. Transporting the doses into rural areas could be simpler, using refrigerated trucks or cold boxes.
But equipment and infrastructure is only half the problem. Local health care workers may not be as aware of the critical nature of cold chain and consequences of temperature excursion on the potency of the vaccine (See my colleague’s eBook on “Cold Chain Qualification: 5 Questions You must Ask Before Shipping High Value Biologics”). Training the health care workers “on the ground” in handling and administration of the doses will be as important as the equipment provided for temperature control.
However, beyond equipment, training, and cold chain expertise, a successful immunization program needs to keep the big picture in mind as well. The October 16 issue of the New England Journal of Medicine highlighted the fact that the biological characteristics of the Ebola virus cannot account for the widespread nature of the current outbreak; this is the 25th known outbreak of Ebola-related hemorrhagic fever, and the devastation of the current epidemic is “more likely to be a result of the combination of dysfunctional health systems, international indifference, high population mobility, local customs, densely populated capitals, and lack of trust in authorities after years of armed conflict…”
Logistical support for a successful Ebola immunization program will mean applying cold chain expertise across a spectrum of interwoven issues that only begin with clearing customs. These supportive measures will require working with a multi-disciplinary team of in-country partners and building trust among people who have been studied by—but not yet benefited from—US biomedical research. We have a lot of work to do.
Download a case study about how Fisher BioServices developed a qualified cold chain transport solution to transport vaccines to Uganda.
This blog was originally posted on the Fisher BioServices Blog. See other articles written by Dan H. O’Donnell on the Fisher BioServices Blog.