Development of a Chemically-defined, Animal Component-free Medium for T cell Culture
As an industry, biopharmaceutical manufacturing has moved away from using animal components in media formulation. This move was largely motivated by the desire for increased consistency, an improved safety profile, and a simplified regulatory pathway. Gene and immunotherapies are now increasingly making their way to clinical trials and once again, there is a desire to move away from media that contain animal-based and other undefined components. Even more important in these therapies is the impact that animal components and their naturally occurring cytokines and growth factors can have on cells.
Recently, I was able to attend a talk on this topic. The presentation, “Optimal T Cell Expansion under Defined Conditions,” was given by Dr. Jessie Ni, Chief Scientific Officer, Irvine Scientific. In the presentation, Dr. Ni described the development of Irvine Scientific’s recently launched PRIME-XV® T Cell CDM, an off the shelf, commercially available chemically-defined, animal component-free medium for human T cell culture.
Drawing on Over 45 years’ experience with Cell Culture Media
Dr. Ni began by describing the work that her group had been doing on developing culture media for T cell subsets. During this work, they spent much time examining the impact of various nutrient components under serum-free, defined conditions. Specifically they looked at the effect of each component on T cell growth and polarization. They also examined how these might work in combination.
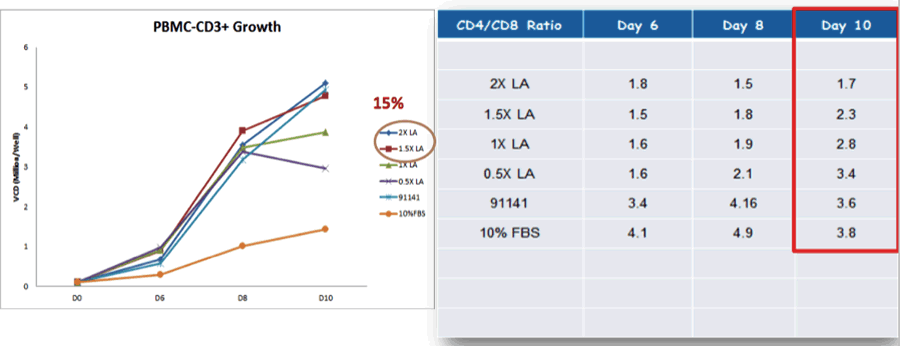
For example, Dr. Ni presented data on how Lipid A (LA) specifically benefitted CD8 T cells Expansion (Figure 1).
Figure 1:
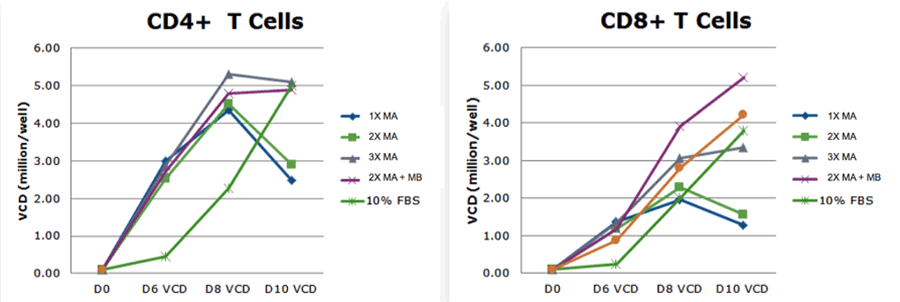
She provided another example looking at the impact of metals. She showed that while Metal A provided a benefit to both CD4 and CD8 T cells expansion, adding metal B provided an increased benefit compared to metal A alone (Figure 2).
Figure 2:
By examining these components in detail and utilizing findings like these, they learned that under serum-free, defined conditions, different media components impact T cell subsets differently. Also optimal expansions of targeted T cell subsets, wanted and unwanted, should and can be achieved through media design and development.
Dr. Ni shared that this type of media was needed in the industry as more gene and immunotherapies make their way to clinical trials. In addition, they also utilized their vast experience in industrial cell culture and Cell Therapy media to meet supply chain and regulatory requirements.
Key Benefits of Chemically-defined and Animal Component-free
Dr. Ni continued the discussion by explaining the key benefits of using a chemically-defined, animal component-free formulation for T cell culture. She began with the scientific benefit of consistency. When using animal components, you can see significant lot-to-lot variability that alters culture performance. By eliminating these components you increase consistency and can obtain results that are reliable and reproducible.
Next she explained that there is better raw material control, which means that you don’t have to pre-qualify multiple lots of animal-sourced raw materials to find the one with matching performance characteristics. In addition, you have increased safety by eliminating animal components and thus a smoother regulatory pathway.
PRIME-XV T Cell Media Performance
She then shared some specific benefits of the Irvine Scientific’s PRIME-XV T Cell Media, including data showing that it outperforms commercially available xeno-free media. (Figure 3).
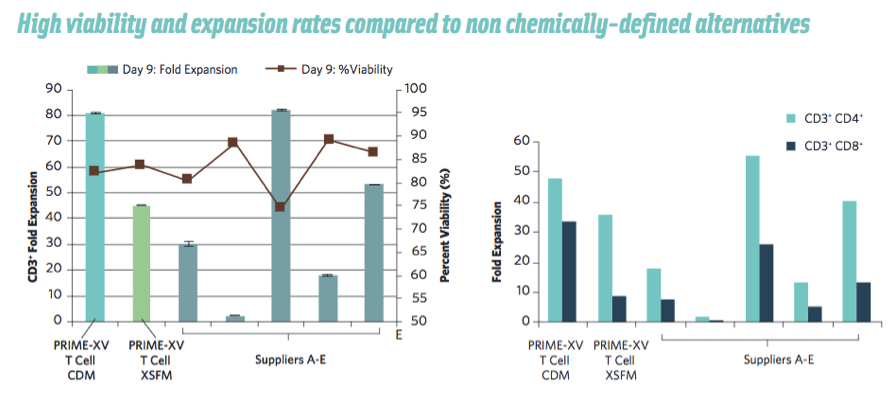
Figure 3. CD3+ T Cells derived from human peripheral blood mononuclear cells, were activated and cultured on treated polystyrene plates in PRIME-XV T Cell CDM or commercially available xeno-free expansion media supplemented with IL-2. After 9 days, the viability and fold expansion of CD3+ T Cells were quantified.
Supports Polarization to Targeted T cell Types
Dr. Ni also described how the new medium was formulated to support polarization to targeted T cell types such as Th1 and T regulatory cells (Figure 4).
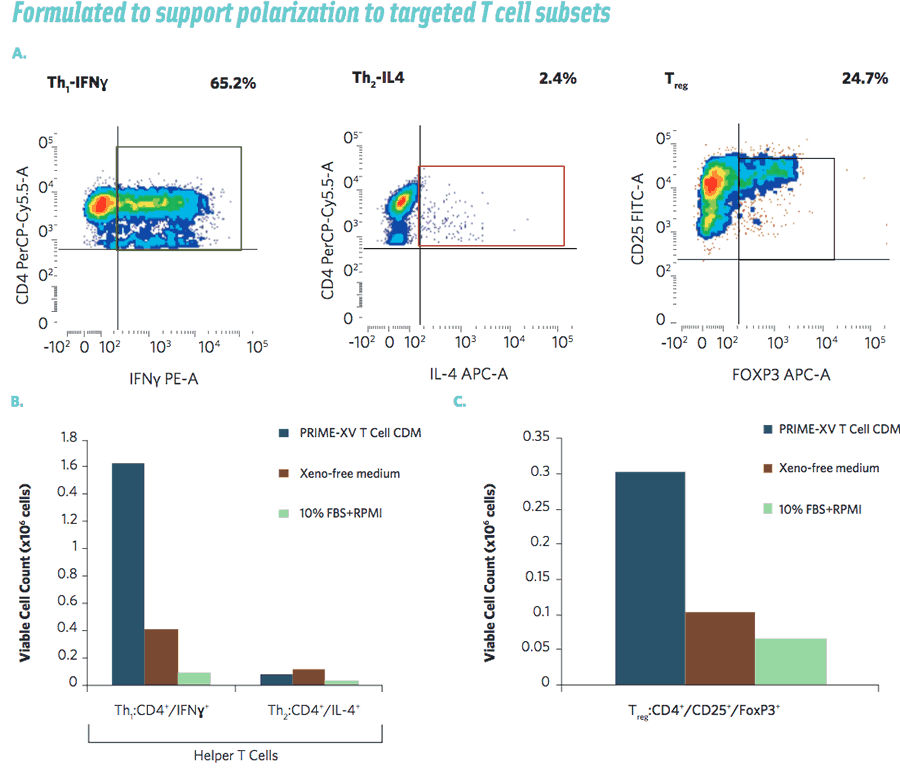
Figure 4: Purified CD3+ T cells were activated using anti-human CD3 and anti-human CD28 antibodies and cultured for 2 days in media supplemented with IL-2. For the polarization of Th1 cells, T cells were cultured for another 72 hours in media containing 5 ng/mL of recombinant human IL-12 and 10 μg/ml of anti-human IL-4 antibodies. For T regulatory (Treg) cell polarization, T cells were cultured for another 72 hours in media containing 50 ng/ml of recombinant TGF-β1 and 6ng/mL of retinoic acid. Flow cytometry analysis was performed to show the representative populations of Th1, Th2 and Treg cells cultured in PRIME-XV T Cell CDM (A); total number of viable polarized CD4+ cells expressing IFNγ or IL-4 (B); and total number of viable polarized CD4+ cells expressing CD25+ and FoxP3+ (C).
Lastly Dr. Ni explained the benefits of using a chemically defined medium in the scale up and commercial manufacture of cell therapies. Eliminating animal and other undefined components minimizes the risk of introducing adventitious agents and simplifies downstream processing. In addition, she explained that Irvine Scientific designed the media with regulatory standards in mind to ease the transition from research to clinical manufacturing. This includes ensuring that the media is manufactured in compliance with cGMP regulations, with complete traceability documentation and an extensive supply chain control program to ensure the highest quality products.
For more product information, please visit PRIME-XV T Cell CDM
Or to contact Irvine Scientific: Tel: +1 949 261 7800 Email: getinfo@irvinesci.com