Electroporation-based Transfection Demonstrates Consistent Antibody Quality and Glycosylation Patterns for Biotherapeutic Product Development
Increasingly, companies are choosing CHO-based transient production of antibodies in early biopharmaceutical development over stable cell line generation. The advantage of using transient transfection is that it takes significantly less time and cost to generate material when compared with the alternative of developing a genetically stable cell line for manufacturing. This is particularly important in drug development where preclinical material is needed quickly in order to make informed go/no go decisions. Having access to preclinical material faster and with less cost can greatly impact the overall drug development timeline.
By initially using transient production, gram quantities of material can be generated quickly and cost effectively, with the plan that if the product progresses further in development, a stable cell line will be generated for larger scale manufacturing. However, for biotherapeutic development to fully benefit from transient production, it must be demonstrated that the transiently produced candidate accurately represents the product that will ultimately be manufactured via a stable cell line. Thus characteristics, such as protein quality and the glycosylation profile must be similar between the transiently produced material and stably produced material.
Product quality and glycosylation are particularly important because post-translational modifications can greatly impact biotherapeutic efficacy and pharmacokinetics. Proteins are often glycosylated, which is a process where proteins are post-translationally modified with the addition of sugar residues. Hence, to maintain the integrity of the candidate selection process, the glycosylation pattern must remain similar between starting material and larger scale manufacturing.
As part of our Boston Biotech Week 2016 coverage, we will be writing about some of the posters presented at the conference. One poster that examined this topic in depth was “Consistent Antibody Quality and Glycosylation Patterns Support the Use of MaxCyte’s Electroporation-based Transfection for Biotherapeutic Product Development,” presented by MaxCyte. In the poster, MaxCyte presents data on protein quality and glycosylation patterns for antibodies and Fc fusion proteins produced transiently as compared to materials produced using a stable cell line. The data presented demonstrate the reproducibility of MaxCyte’s transient expression system and that transiently produced proteins mimic product qualities of stably produced proteins. In addition, long term stability of titers and protein quality in stable cell lines generated via MaxCyte electroporation were presented.
Highlights of the poster include:
CHO-based Transient Production Resulted in High Quality Proteins
The MaxCyte electroporation-based platform is flexible able to efficiently transfect a variety of CHO cell lines including CHO-S, CHO-K1, CHO EBNA, CHO-K1SV, and CHOZN®. High titers, consistent protein quality and reproducibility were demonstrated when compared with other transfection methods (Figure 1, 2)
CHOZN®: Gel Analysis of Protein Quality and Titer
50Kda Heavy Chain and the 25Kda Light Chain Are Clearly Seen
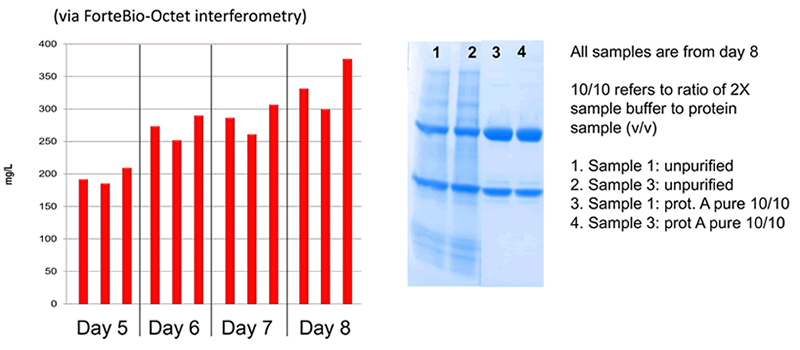
MX CHOK1-SV: Low Degradation Fc Production
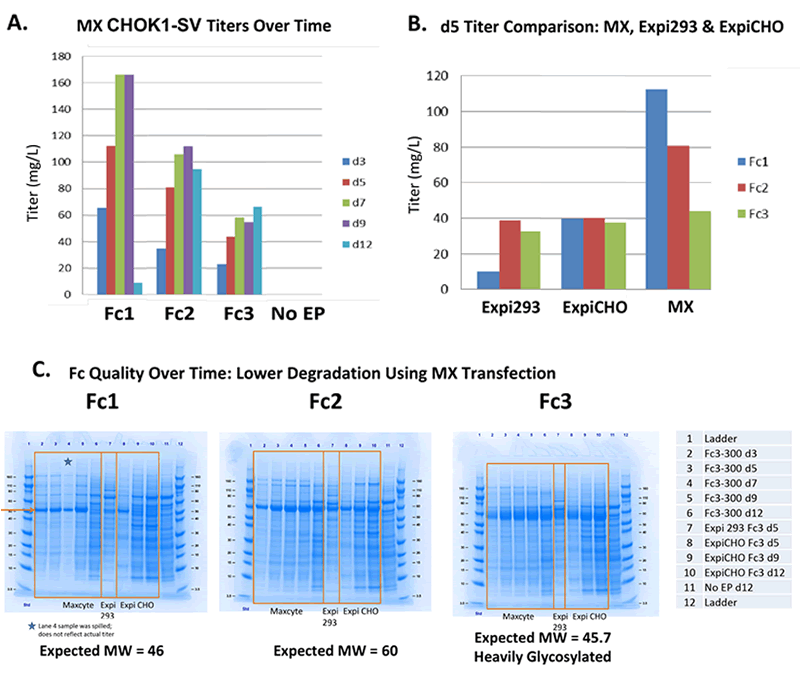
MaxCyte Outperforms Expi293 & ExpiCHO
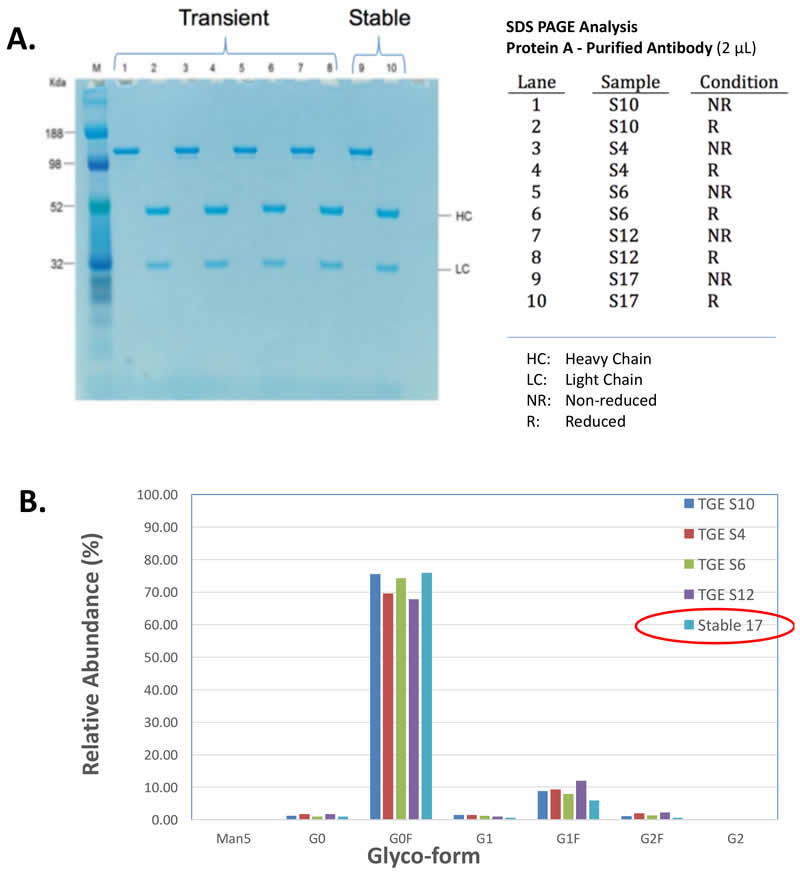
Transient Produced Material Was Consistent with Stably Produced
MaxCyte electroporation was used to transiently produce protein and also to generate stable cell lines that expressed the same protein. Then the transiently expressed protein was compared side-by-side with the stably produced protein. from eachwere then expressed transiently or stably produced were then compared side-by-side. The transiently produced proteins demonstrated similar protein characteristics and glycosylation patterns to the proteins produced using the stable cell lines (Figure 3). Thus, supporting the use of transiently produced protein in early stage biotherapeutic development.
Equivalent Antibody Quality & Glycosylation
Similar IgGs Produced via MaxCyte Transient Transfection & Stable Cell Lines Generated Using MaxCyte Transfection
MaxCyte Electroproation Rapidly Generates High-producing Cell Lines That Maintain Titer, Quality and Glycosylation Over Time
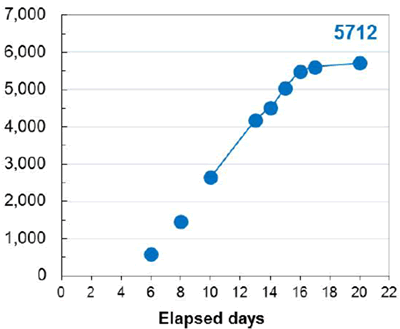
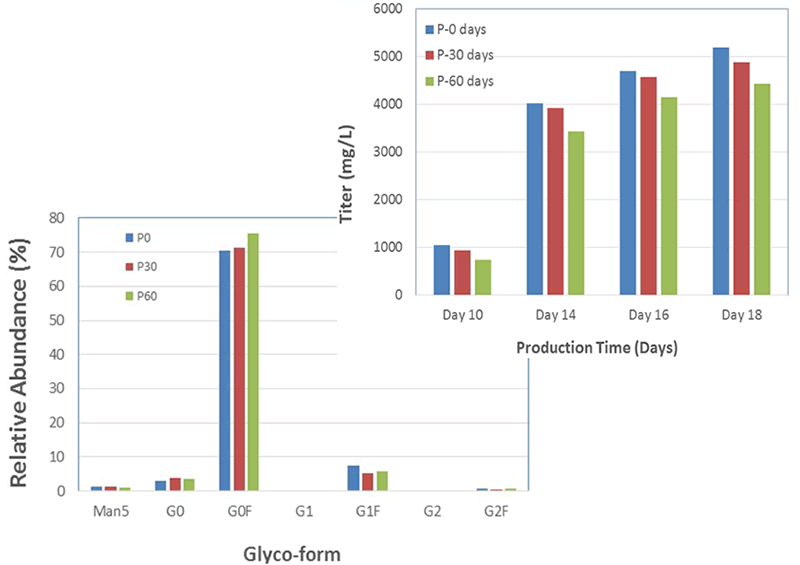
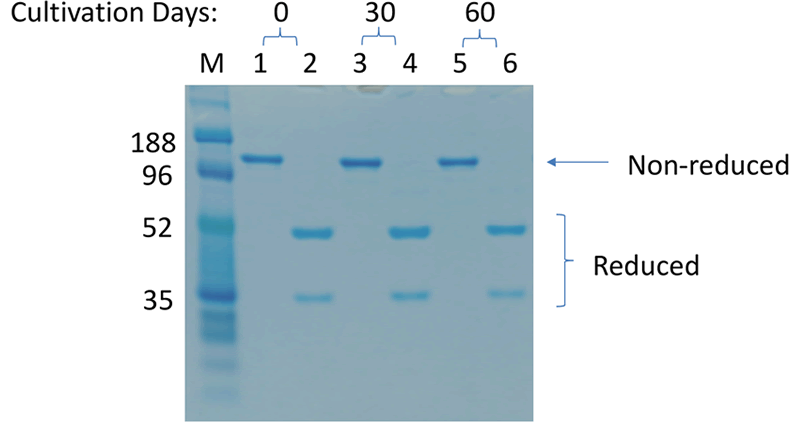
An additional benefit of using this system was the efficient generation of stable cell lines following electroporation. Due to the high CHO cell viability post electroporation, it was possible to generate stable pools and develop stable clones with yields >5.7 g/L in just 6 weeks. Less than 500 clones were needed for screening to identify a high-yield stable cell line. Furthermore, these stable cell lines maintained protein titers, quality and glycosylation patterns over 90 days (Figure 4).
Stability of MaxCyte-produced Cell Lines
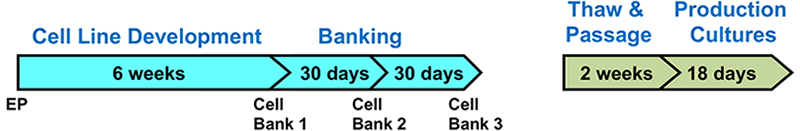
A. Top Producing Stable Cell Line 6 weeks Post EP
B. Titer & Glyco-form Analysis
C. Protein Quality
For more data and full details of the highlights discussed here, please click on the poster below to view in full size.
