
Enhancement of transient transfection with serum-free and blood-free transferrin
Abstract
Methods to enhance transient transfection of adherent cells have identified many different ligands that improve plasmid uptake into cells. Of these ligands, serum-derived transferrin is well-known to improve transfection efficiency of adherent cells. However, to date, a serum-free and blood-free transferrin has not been tested for improvements to transfection efficiency. We have thus created a protocol for the improvement of transfection efficiency and viral titer without the need for blood-derived transferrin. Excess transferrin does not negatively impact transfection, and the incorporation of a blood- and serum-free, recombinant human transferrin supports high transfection efficiency and viral titer in a chemically defined, blood-free medium.
Background
Transient transfection relies on the spontaneous fusion of plasmid DNA with the cell membrane and the subsequent endocytosis and trafficking to the nucleus in order to achieve transient gene expression. Improvements made to polymers or lipids have resulted in numerous commercially available products for highly efficient transfection. However, these products are still subject to fusion with the cells and the ability of cells to endocytose foreign DNA.
Methods to improve a cell’s ability to take in plasmid DNA have identified that transmembrane receptors can serve as ports of entry for the internalization of extracellular DNA. While there are thousands of receptors that can be present on a cell, the transferrin receptor 1 (TfR1) is ubiquitously expressed, as every cell requires extracellular iron for health and growth. Transferrin has been identified as a therapeutic agent for the delivery of nanoparticles and small molecules due to the ability of transferrin to be covalently linked and also as a natural carrier of molecules for entering a cell through its receptors.1
Given the success of transferrin as a molecular carrier, the use of transferrin has still relied on serum-derived transferrin from either bovine serum or human serum. Both of which are subject to numerous disadvantageous factors such as adventitious agents present in serum, constraints on the supply-chain, or increasing cost due to demand. We have therefore developed a protocol for the use of a recombinant human transferrin that is not derived from serum or animal blood.
While excess transferrin in transfection has already been identified to increase transfection efficiency,2 we show that blood-free and serum-free transferrin added in excess during the transfection of adherent cells improves the transfection efficiency compared to low levels of transferrin and serum-derived transferrin. We also show that the inclusion of transferrin in transient transfection for viral production enhanced functional viral titers for lentivirus. Our data suggest that serum-free and blood-free transferrin can be used as a carrier for nucleic acids and potentially other molecules for trafficking molecules into cells.
Methods
The methodology, including cell types, passaging conditions, materials, flasks, plating information, transfection, and viral tittering, is covered in greater detail in the full white paper. Please see Enhancement of Transient Transfection with Serum-free and Blood-free Transferrin.
Discussion
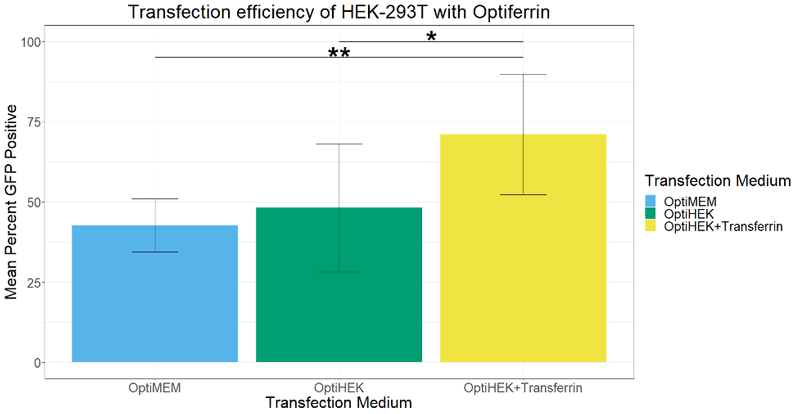
In standard conditions, OptiPEAK HEK293t® achieves equivalent transfection efficiency compared to transfections performed under serum conditions complexed with OptiMEM. The average percentage of GFP positive cells complexed with OptiMEM and grown in DMEM + 10% FBS was 42.6% ± 8.1% and the average with OptiPEAK HEK293t was 48.1% ± 20.1% (Figure 1, difference in means is not statistically significant). Including excess transferrin (Optiferrin®) in the transfection medium of OptiPEAK HEK293t raised the average percent GFP positive cells significantly to 71.1% ± 18.8% compared to DMEM + 10% FBS and OptiPEAK HEK293t without excess transferrin (Figure 1, p < 0.05 by Student’s t-test).
These results indicate that excess transferrin does not negatively affect transfection and can enhance transfection efficiency of HEK-293T cells in serum-free, chemically defined conditions.
To determine if transferrin enhancement of transfection efficiency correlated with higher viral titers, we performed transient transfection with lentivirus plasmids for viral production with 293T cells. Excess transferrin in the transfection step for viral production led to an increase in functional viral titer for lentivirus compared to Complete OptiPEAK HEK293t without excess transferrin (Figure 2). Lentivirus production showed similar trends to transfection efficiency assays with a GFP reporter. Second generation lentivirus vectors were produced using a three plasmid system, and total lentivirus vectors were harvested 48 hours post transfection. Lentivirus plasmids complexed with OptiMEM or OptiPEAK HEK293t showed near equivalent functional titers, with the average titer from plasmids complexed with OptiMEM and then cells grown with DMEM + 10% FBS was 1.79 × 107 Infectious units (IFU)/mL ± 1 × 107 IFU/mL (Figure 2).
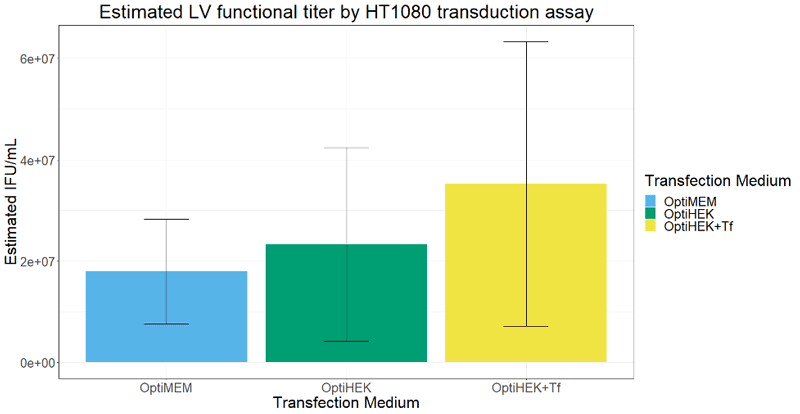
Plasmids complexed with OptiPEAK HEK293t and grown in chemically defined conditions with OptiPEAK HEK293t yielded average titer of 2.3 ± 1.9 × 107 IFU/mL (Figure 2, difference in means is not statistically significant). Plasmids complexed with excess transferrin in OptiPEAK HEK293t showed a log-fold increase over OptiPEAK HEK293t in average functional titer, with an average of 3.53 ± 2.8 × 107 IFU/mL (Figure 2).
Together, these results demonstrate that excess transferrin does not inhibit DNA complexation and in fact improves functional lentivirus titer compared to chemically defined conditions without excess transferrin and improves functional titer compared to serum-derived transferrin in OptiMEM. The improvements shown here with excess transferrin suggest that 293T cells may be more rapidly endocytosing plasmid DNA from the complexation medium. Another hypothesis for the improvements shown here is that the plasmid DNA is better protected within the cell as it is trafficked to the nucleus.
Regardless of the mechanism, a serum-free, blood-free, recombinant human transferrin is able to achieve high transfection efficiency, and high viral titer, suggesting that blood-free transferrin has high functionality in a chemically-defined medium.
To learn more, please see the full whitepaper Enhancement of Transient Transfection with Serum-free and Blood-free Transferrin.
About the Author
 Sofia Pezoa, PhD, Cell Culture Scientist, InVitria
Sofia Pezoa, PhD, Cell Culture Scientist, InVitria
Sofia Pezoa is a cell culture scientist for InVitria where she works on product development for the manufacturing of virus based vectors for gene therapies and vaccines. Prior to joining InVitria, Sofia earned her PhD from the University of Colorado Anschutz in the Cell Biology, Stem Cell, and Developmental Biology graduate program.
Footnotes
-
1. Daniels, T. R., et al. (2012). "The transferrin receptor and the targeted delivery of therapeutic agents against cancer." Biochim Biophys Acta 1820(3): 291-317.
-
2. Tros de Ilarduya, C. and N. Duzgunes (2013). "Delivery of therapeutic nucleic acids via transferrin and transferrin receptors: lipoplexes and other carriers." Expert Opin Drug Deliv 10(11): 1583-1591.