
Publication review: Formulation and production of a blood‐free and chemically defined virus production media for Vero cells
Developing serum-free medium candidates for Vero (African monkey kidney epithelial) cell culture is both a topical and significant breakthrough, as there is an increasing intersection between vaccine development, raw material availability, and regulatory pressures for enhanced quality and safety. Leveraging recombinant proteins in cell culture has garnered increasing interest as a means to enhance the safety and consistency of virus production during manufacturing leading to improved virus-based vaccines and therapeutics.
Vaccines are a versatile class of prophylactics that prime the immune system to prevent infection by diseases through exposure to a very small amount of the pathogen, in many cases a virus, that has been weakened or killed to elicit an immune response through antibody production and the creation of immunogenic memory. If the immune system encounters the pathogen again, it will readily recognize the antigens and mount an attack before the pathogen has a chance to spread and cause sickness. Widespread vaccination programs are therefore essential to maintaining the health of the global population, which necessitates the ability to manufacture vaccines in large quantities.
Vaccine Manufacturing
The large-scale manufacture of live attenuated vaccine and inactivated vaccines has traditionally involved producing viruses within host cells such as embryonated chicken eggs and primary and diploid cell culture systems. However, increased demand for vaccines has engendered a shift in the industry to more robust large‐scale methods that utilize mammalian continuous cell lines (CCL). Vero cells are the best characterized, most widely used CCL system and have been a workhorse for human vaccine manufacturing for nearly 40 years.
Optimizing Media for Vaccine Production
Traditionally, serum-based culture media are used to expand Vero cells for virus production, but more stringent guidelines from regulatory bodies to increase safety profiles of cell-based products has vaccine developers using serum-free, plant hydrolysate-based media. However, a major constraint with plant hydrolysates is that they are complex, undefined mixtures that can introduce unwanted variability when used in vaccine manufacturing. To circumvent the lack of chemical definition in virus production media, Alfano et al. published a paper describing a chemically defined, serum-free media formulation known as OptiVERO®, which uses recombinant human transferrin and recombinant human albumin for virus production in Vero cells in both 2D and 3D culture formats.
The researchers first used a design of experiments (DOE)-based approach to create media formulations. With Vero cell growth used to assess the media formulation efficacy, Plackett–Burman matrix designs were used to screen the various media formulation combinations with the goals of removing biologically inert or detrimental compounds as well as identifying the optimal concentration range of the necessary media components. From these early studies, a single formulation, referred to as OptiVERO, was selected based on formulation complexity and performance for further cell growth kinetics and virus production experiments.
To evaluate the efficacy of OptiVERO, cell proliferation studies in 2D and 3D formats as well as virus production capacity were evaluated in comparison to serum-containing media, EMEM‐10% FBS, and a commercially available serum-free formulation containing plant hydrolysates, VP‐SFM (Thermofisher Scientific). In 2D cell proliferation studies, Vero cells grown in OptiVERO showed similar morphology and demonstrated comparable doubling times to EMEM‐10% FBS at 42.99 ± 5.68 and 40.07 ± 4.16 doublings in the 50 days, respectively, which were both higher than that observed for VP-SFM at 36.15 ± 4.44 doublings in the same time period (Figure 2, from publication).
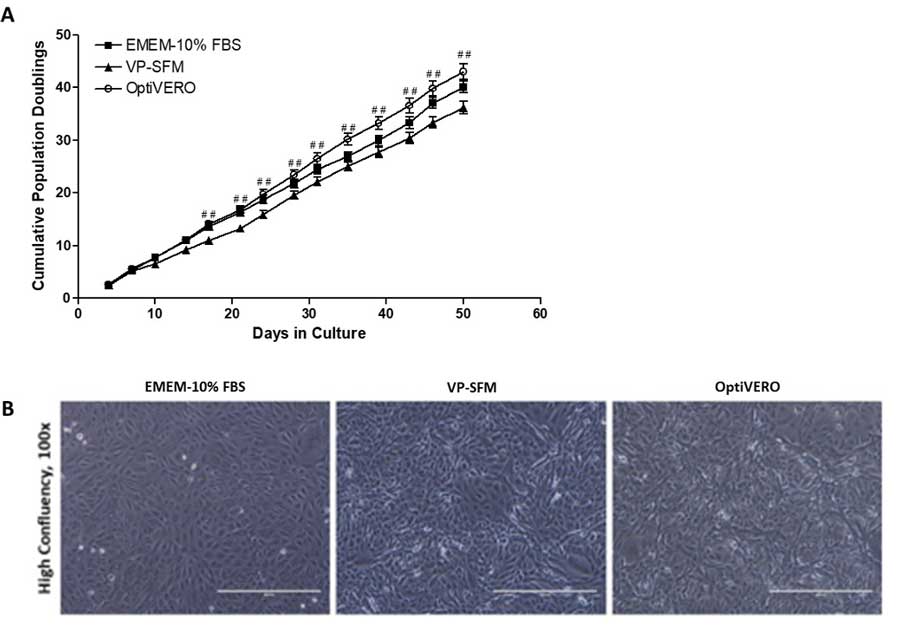
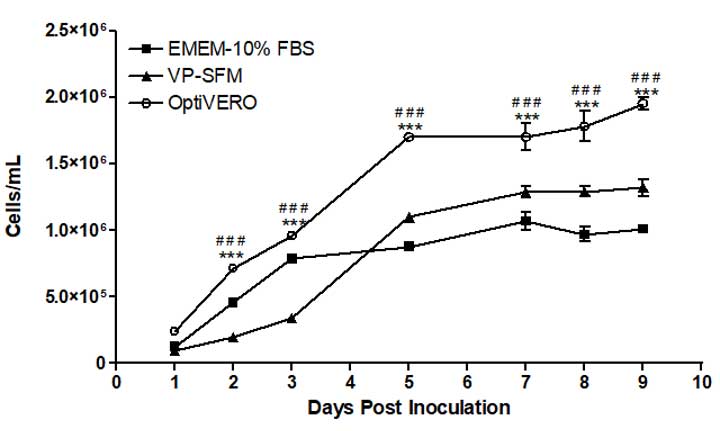
The results obtained from proliferation studies using plastic microcarrier 3D cultures were similar to that of the 2D system where Vero cells cultured in OptiVERO or EMEM‐10% FBS expanded rapidly within the first three days of spinner culture (Figure 3, from publication). In fact, the cell numbers in OptiVERO spinner were between 50 – 70% higher than the controls from Day 2 to Day 9 after cell seeding.
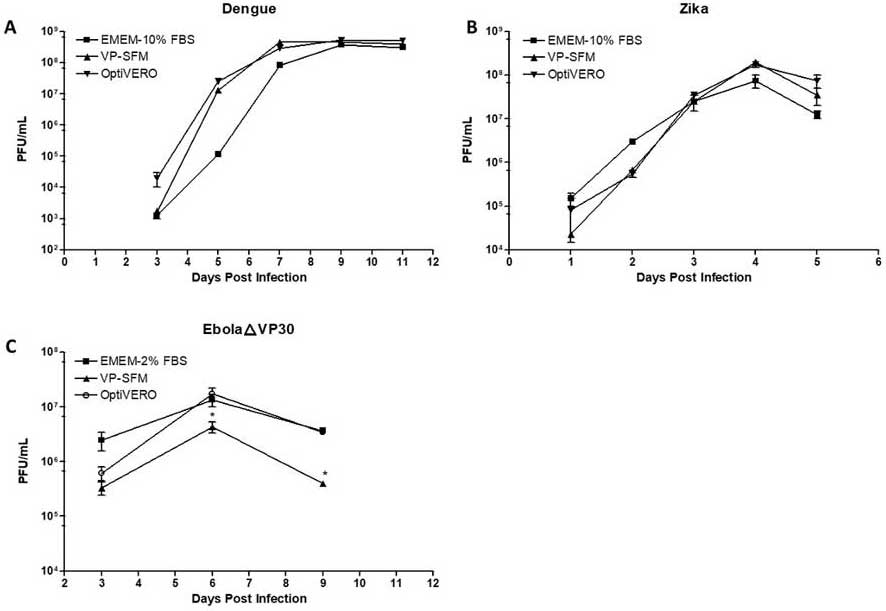
While these results were promising, the most critical determinant of success is the ability of the Vero cells to produce virus at high titers. Virus production efficacy was tested in OptiVERO and the two-control media using three virus strains: DENV‐2 16681, ZIKV, and EbolaΔVP30. The production of two different flaviviruses, DENV‐2 16681 and ZIKV PRVABC59 in 3D microcarrier spinner cultures was comparable across all three media. In the case of EbolaΔVP30‐infected Vero cells culture in 2D T‐flasks, titers of 1.35 x 107 PFU/mL and 1.74 x 107 PFU/mL, in EMEM‐2% FBS and OptiVERO, respectively, were observed on Day 6 of post-viral infection (Figure 4a and b, from publication). Notably, the VP-SFM was not as supportive for EbolaΔVP30 expansion, demonstrating a 1-log reduction in virus titer compared to the other media. (Figure 4c, from publication).
The safety issues surrounding FBS usage for cell-derived products is complicated by the fact that component proteins in FBS are known to be critical drivers of Vero cell growth, survival, and, ultimately, virus production. Plant‐derived di‐ and tripeptides have been investigated as potential replacements to bovine serum‐derived proteins, but considerable variability exists between different lots of these complex mixtures, which impacts virus production efficacy. In the OptiVERO formulation, Alfano et al. replaced the serum components with recombinant human proteins that renders OptiVERO media as both serum-free and chemically defined.
Successful Alternative to FBS in Vero Cell Culture
Overall, the data presented in this publication demonstrate that OptiVERO performs as well as (or better than) FBS-containing media in cell proliferation and virus production and also outperforms the plant hydrolysate-containing VP-SFM media.
The successful replacement of serum (or plant hydrolysate additives) with recombinant proteins to produce a chemically defined, serum‐free virus production media enhances the safety and consistency of virus production during manufacturing, which will improve virus-based vaccines and be more suitable for other novel clinical applications.