
Future Proofing Upstream Bioprocess Operations
 This article was originally published in the eBook
This article was originally published in the eBook“ Future proofing Bioprocessing from Upstream to Downstream”.
You can download all the articles in the series, by downloading the eBook.
In the early days of upstream biotherapeutic manufacturing, processes looked much different than biologic production today. Most early manufacturing runs were conducted as batches and ran for seven days with yields around 100 mg/liter. Then bioreactors began to be used and run times moved to 10-14 days with media and nutrients being replenished during the run. Now commercial biologics manufacturing utilizes specialized bioreactors, media and nutrients, and cells are monitored continuously.
As we look toward the future of upstream bioprocessing, the industry needs to remain mindful of the next wave of process advancements and manufacturing challenges. The goal is to design systems that are future proof and can handle advancements and any potential setbacks. It is also important to design fit-for-purpose systems that are tailored to an end users’ specific needs, are easily reconfigurable, and can evolve with the workflow to incorporate technological innovation and respond to changes in supply or demand.
In this article, we will look at a few examples of how, with careful design, upstream bioprocessing solutions can be flexible, fit-for-purpose and future proof.
Addressing Supply Chain Constraints
The industry is currently experiencing significant equipment and raw material shortages, largely due to the impact of the COVID-19 pandemic, but also due to an increase in single-use product demand.¹ However, some materials are prone to shortage, and were diffcult to source prior to the pandemic, including some key high-purity polymers.² As a result, end users can face long delivery times, sometimes up to fourteen months, for certain consumables.³ Furthermore, many equipment vendors have single source supply chains because of their proprietary solutions, which also impacts the ability to increase supply quickly, as does facility and workforce constraints.³
Supply challenges can be addressed in the near term by providing equipment designs that are less proprietary and more open or agnostic. This way multiple vendors can be leveraged to provide a solution, thus permitting quicker deliveries. Brand-agnostic systems provide end users the freedom to work with the companies and products that best meet a workflow need. This approach can also help mitigate supply chain issues, as process equipment can have the flexibility to use components from different vendors and brands.
Agilitech provides brand-agnostic systems that can be reconfigured to use filters, sensors, and other components from virtually any manufacturer brand. As a result, Agilitech is not limited to the same supply chain constraints that other vendors are. If there is an issue with delivery of a specific component, they can pivot immediately to a different vendor with quicker delivery times without any effect on the design or the delivery of the system.
Case Study – Avoiding Supply Chain Delays with a Brand-agnostic Approach
Recently Agilitech has had to work with several customers to overcome the challenge of supply chain shortages. In many instances, customers are requiring specific timeframes for needed equipment and Agilitech will search for and locate different components that could be substituted to meet deadlines. Specifically, one customer was looking to purchase a depth filtration skid that was 30 to 40 weeks out on delivery. Working with a different platform, Agilitech was able to cut that delivery time in half and still meet all the requirements that the customer had for delivery. It is important to look at what is happening in the industry and which components are causing delays, then anticipate shortages and pivot to other suppliers or product types to meet timelines.

The Agilitech benchtop bioreactor system is adaptable to microbial and cell culture configurations. With a brand-agnostic design, it offers the flexiblity of choosing your own vessel configuration, preferred sensors, as well as the automation and control platform.
Another example was when a customer had a system that was specified for a certain type of programmable logic controller (PLC) components. By the time that the purchase order was issued, the lead time on these components had exploded. Agilitech was able to substitute different PLC components that met all specifications and were available to meet project timelines. This required Agilitech to quickly re-design the internal enclosure components and connections, which they completed in time to meet overall project deadlines.
Managing Technology Incompatibility and Platform Limitations
One obstacle to creating a fully optimized workflow is technological incompatibility. While customizability of a platform process is often the primary goal, the reality is that customizing an existing platform has limitations.
Market-leading suppliers offer a range of platform systems with some flexibility within their platform of products. However, full flexibility of these products is often limited as the equipment itself is not always adaptive to unique process needs and typically do not integrate products and/or components outside of brand.
There is a need in the industry for more flexible solutions that can be tailored to specific bioprocessing needs, as well as solutions that can adapt and evolve as process requirements change. Agilitech offers a different take on the platform approach by providing systems that can be tailored to specific end user needs and can easily integrate into fit-for-purpose workflows. It might seem like this level of customization would be cost-prohibitive, however front-end planning and collaborative design is typically no more costly than other available products, but provides significant benefits to the overall workflow.
Case Study – A Fit-for-purpose Solution
In this case study, a fit-for-purpose solution was developed for a customer. The customer’s existing process consisted of feeds using theoretical volume calculations based on the peristaltic pump, thereby assuming that each revolution of the pump equates to a certain volume and then tracking the revolutions.
What the customer needed was a more accurate picture of the amount of each feed going into the process. They wanted each of their feeds to be on an individual load cell scale so that they could measure the weight of each feed bottle before and after to calculate total feed volume.
At first, the customer discussed their interest in putting four bottles on one scale and then delineating how much is being used at each bottle. This was possible, provided they were not planning on running the pumps at the same time. However, they wanted to be able to run the pumps simultaneously across multiple bottles. Agilitech then developed a system of eight load cells, four for each bioreactor, with a bottle holder on each load cell that could hold the specified bottle sizes. Four load cells for each bioreactor are tied into a single weight transmitter that is able to delineate the eight signals and send them in one condensed cable over DeviceNet™ to their existing data collection solution.
Once the solution was identified, Agilitech needed to provide this in a compact system to conserve limited bench space. This resulted in placing the system on a stand above the pump towers, allowing the entire system to sit above the bench and leaving it open for other work.
Designing a Workflow Now and for the Future
Future proofing a manufacturing system ensures that it evolves with the company. To do this successfully, it is important to understand short- and long-term manufacturing goals, as well as current constraints. It is also key to reduce capital spending up front while allowing companies to purchase only what they need at the time, but with the flexibility to grow the system as the company grows.
Case Study – Future Proofing a Switch to Automation
In this instance, the customer was using depth filtration in their current harvest process and this was a process they ran manually. The customer needed to run up to three filter trains in parallel. They would wait for one filter to clog, then they would transition to the next one and so on.
In addition, the system was originally designed with three inlet valves to determine which solution is being pumped, but with the new desire to run three filter trains, they needed more granular control of the destination. So, the three inlet valves were repurposed as outlet valves.
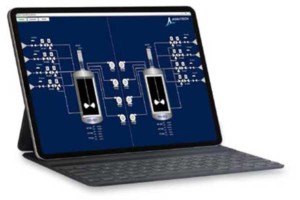
Bioreactor system controls with flexibility to support manual operation to full batch control are key to a future-proof process. Agilitech delivers systems with DeltaV™, Rockwell Automation®, Inductive Automation Ignition®, Wonderware®, or other control system based on customer need. An easy-to-use controller interface offers added convenience by bringing the most commonly used functions and data to the top layer of the screen.
Another custom feature of this project was the customer’s desire to run multiple size tubing ID’s with the skid. Agilitech designed the skid to be able to swap between 1-inch and 3/4-inch tubing at the valves and sensors.
By using Agilitech’s multipurpose filtration skid, originally designed for downstream operations, and configuring it to fit this specific process, the customer was able to move their depth filtration process from a completely manual process where operators were manually controlling pump speed, monitoring flows, and pressures to a system that was fully automated. The Agilitech solution provided an automated system from flushing to filtration to post-use flushes and filtrate and blowdowns.
Because the customer is a CMO running multiple products through their facility each year, they needed a solution that would be applicable for the current product and flexible enough to handle any other filtration systems that they could envision using in the future. Agilitech designed the automation component to be quite flexible to accommodate any future products as well.
Conclusion
A common theme across all case studies was collaboration. There needs to be good communication between the biopharmaceutical companies and suppliers to ensure that the process created exactly fits the purpose for which it was designed, that the process is future proof and able to evolve with the company, and that the required equipment and consumables will be able to be timely sourced even during supply chain shortages.
Agilitech utilizes a project implementation process that encourages and supports this collaboration. The process of creating a 3D model and design prior to the proposal phase requires customers to be involved early. This involvement continues after the project starts by engaging customers in the review of functional specifications, graphics and dry software runs prior to factory acceptance testing (FAT) to ensure all needs are met. Instead of issuing a purchase order and waiting to receive updates on the status of delivery, with Agilitech customers are partners in the process ensuring that they receive the exact product that meets their needs.
 This article was originally published in the eBook
This article was originally published in the eBook“ Future proofing Bioprocessing from Upstream to Downstream”.
You can download all the articles in the series, by downloading the eBook.
Footnotes
-
1. Sanders, P. (September 21, 2021). Future-proof Bioprocesses: Flexible Single-use Technology that Adapts to an Evolving Industry. Accessed September 1, 2022 : Retrieved from https://downstreamcolumn.com/future-proof-bioprocesses-flexible-single-use-technology-adapts-evolving-industry/
-
2. Rader RA, Langer ES, Jhamb K. COVID-19 Impact on Bioprocessing: Accelerating Trends and Long-Term Impact of Novel Coronavirus-19 on Biomanufacturing and Bioprocess Supply Chain. BioPlan Associates: Rockville, MD, June 2020; https://bioplanassociates. com/wp-content/uploads/2020/07/Covid-19-Impact-on-Bioprocessing-White-Paper-BioPlan-20200605.pdf.
-
3. Sanders, P., & Sargent, B. (Oct. 28, 2021) Addressing the increasing demand for single-use technologies and supply chain shortages with future proof systems. Accessed September 1, 2022 : Retrieved from https://downstreamcolumn.com/addressing-increasing-demand-single-use-technologies-supply-chain-shortages-future-proof-systems/.