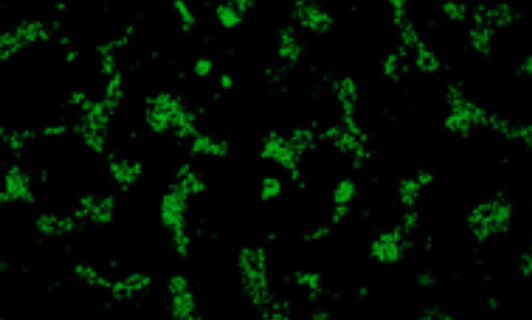
Generation of Neural Stem Cells from AlphaSTEM Cultured Pluripotent Stem Cells
Introduction
Patient-derived induced pluripotent stem cells (iPSCs) offer exciting potential for disease modeling, regenerative medicine, and therapeutic development. In order to fully realize the potential of iPSCs, it is critical to develop culture and differentiation methodologies that result in the most biologically relevant terminal cells (e.g. Neurons).
Minerva Biotechnologies is the first company to generate human naïve state induced pluripotent stem cells (iPSCs) using a single, naturally occurring human stem cell growth factor, NME7AB. Naïve stem cells have several advantages over current stem cells, called ‘primed’ state cells. Scientists believe that because these earlier stem cells have a “clean slate”, they are more easily directed to develop into functional mature cells. Using Minerva’s AlphaSTEM™ Culture System, differentiation efficiency is increased and the resulting cells have enhanced functionality.
Materials and Methods
In this study, iPSCs were expanded in the AlphaSTEM™ Culture System which generates superior naïve stem cells that can be enzymatically passaged as single cells, expanded 10X in 4 days, and are karyotypically stable. The cells then underwent neural induction using the PSC Neural Induction Medium from Thermo Fisher Scientific. The PSC Neural Induction Medium is a serum-free medium that provides high efficiency neural induction of human pluripotent stems cells (PSCs) in only 7 days.
Culturing iPSCs using AlphaSTEM™ Naive hPSC Medium
AlphaSTEM™ Naïve hPSC Medium is a serum-free and feeder-free medium formulated for the generation and expansion of naïve pluripotent stem cells.
Results
Naïve stem cells do not have DNA methylation marks that commit the cells to certain developmental decisions. The result is differentiation of cells grown in AlphaSTEM™ Naive hPSC Medium is superior to that of FGF grown cells in terms of quality, quantity and purity.
Increased Differentiation Efficiency
AlphaSTEM™ Naive hPSC Medium increases directed differentiation to neural cells by 150X over FGF media. When AlphaSTEM™ Differentiation Inducer is used, the increase is 250X.
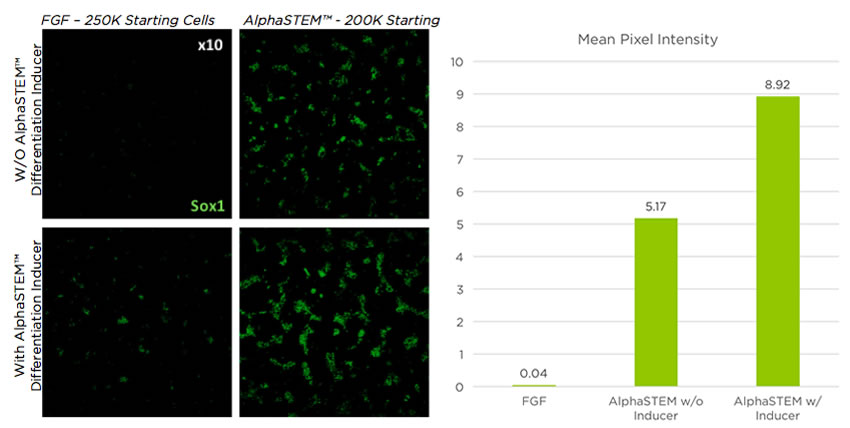
Improved Marker Expression
Neural stem cells generated from AlphaSTEM™ cultured stem cells have increased expression of neural markers. When AlphaSTEM™ Differentiation Inducer is used, the expression is further increased.
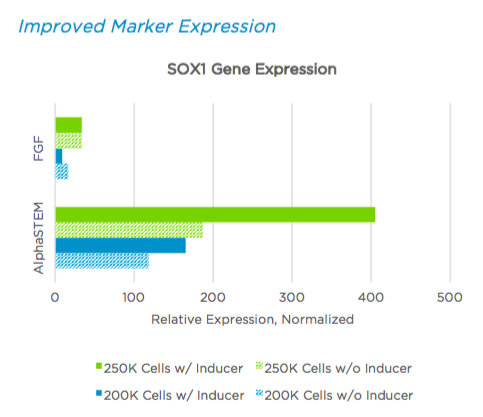
Increased Cell Yield
AlphaSTEM™ Naive hPSC Medium increases final NSC yield compared to FGF based media. Data shows fold expansion during induction protocol was independent of starting cell number.
Conclusions
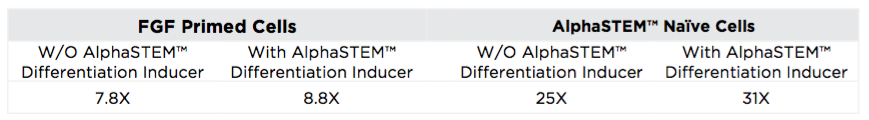
In this study, we demonstrated that using this system results in more efficient neural induction with fold expansion increasing to 25X compared to 7.8x (>300% increase) and a population of cells with 12-fold higher expression of SOX1 at Day 7 compared to the same number of starting FGF source cells. The addition of AlphaSTEM™ Differentiation Inducer further increases the yield giving a 4-fold increase in total cell number and an increase in the number of SOX1 expressing cells by 2-fold.
The Minerva AlphaSTEM™ Culture System was developed to provide a superior alternative to FGF- based cell culture. Using AlphaSTEM™ Naive hPSC Medium, iPSC generation is simpler, cell expansion is faster, differentiation efficiency is increased and the resulting cells have enhanced functionality.
For protocols and additional information, please see: www.minervabio.com/products/stem-cell-reagents/
Footnotes
-
1. A Primitive Growth Factor, NME7AB , Is Sufficient to Induce Stable Naïve State Human Pluripotency; Reprogramming inThis Novel Growth Factor Confers Superior Differentiation. Carter MG, Smagghe BJ, Stewart AK, Rapley JA, Lynch E, Bernier KJ, Keating KW, Hatziioannou VM, Hartman EJ, Bamdad CC, Stem Cells. 2016 Apr;34(4):847-59.
-
2. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, Zhan M, Davis J, Bharti K, Zeng X, Rao M, Malik N, Vemuri MC, Stem Cells Transl Med (2013) 2:862-870