
GenScript Real-time PCR (TaqMan) Primer and Probes Design Tool
Real-time PCR is a powerful molecular biology technique used in various fields that enables the quantification and amplification of specific nucleic acid sequences in real-time. The TaqMan assay is a popular real-time PCR method that employs fluorescent probes to detect and quantify DNA or RNA targets in samples that contain only a few copies, making it valuable in applications ranging from clinical diagnostics to genetic research. The PCR reaction relies on oligonucleotide probes designed to hybridize specifically to the target sequence and are composed of a fluorescent reporter dye and a quencher dye at the 5′ and 3′ ends, respectively. The primers flanking the target sequence serve as the starting point for the Taq polymerase for synthesis of the template.
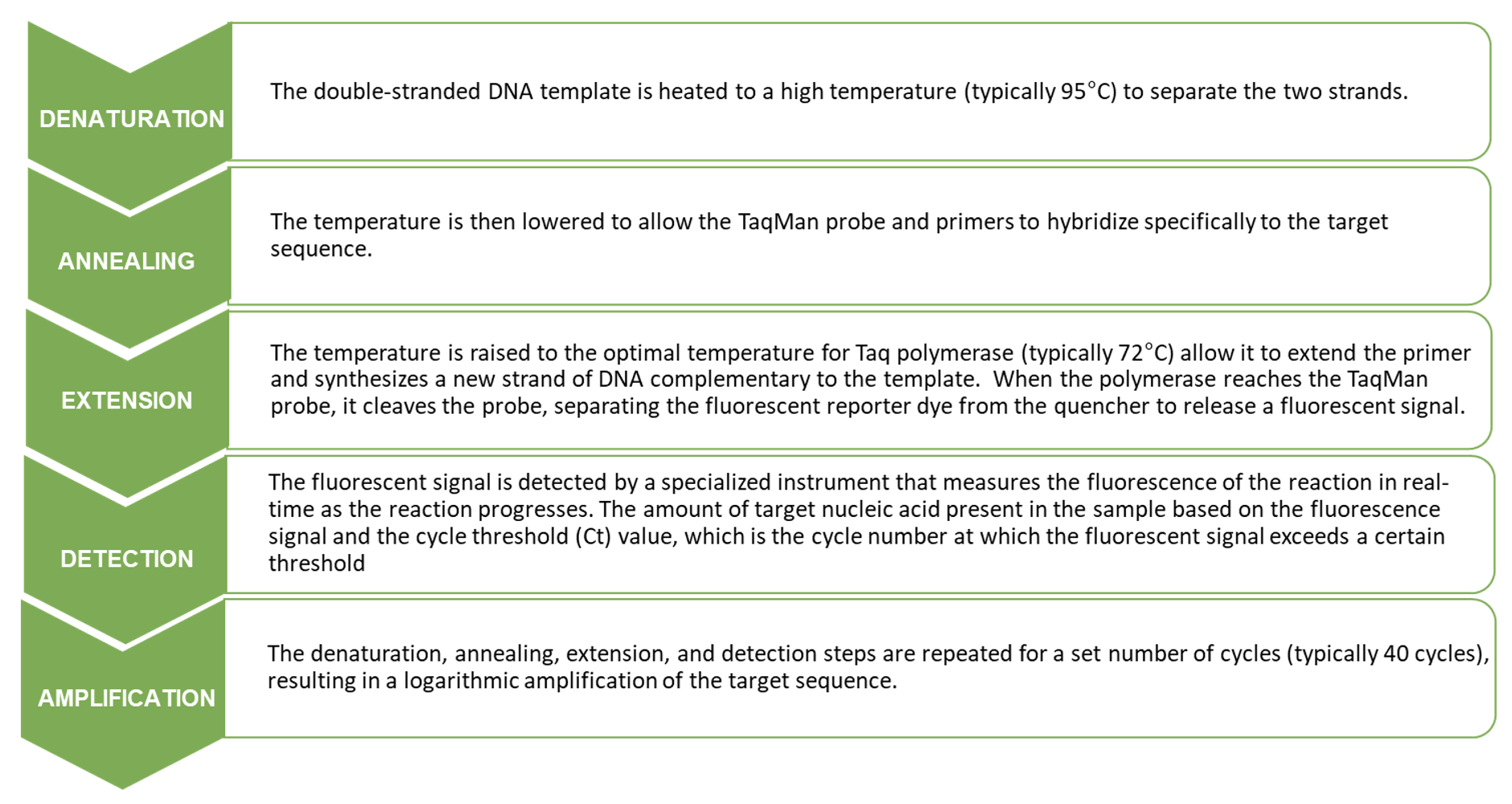
During the PCR reaction, a fluorescent signal is generated when the probe is cleaved by the Taq polymerase during amplification. The fluorescence produced is measured in real time as the reaction progresses and is proportional to the amount of target DNA or RNA in the sample. The more copies of the target sequence present in the reaction, the more TaqMan probes are cleaved, resulting in a higher fluorescent signal. The key steps in a TaqMan assay are shown below where the nucleic acid template is denatured, the TaqMan probe and primers are annealed and extended by Taq polymerase, followed by cleavage of the TaqMan probe, detection of the fluorescent signal, and amplification of the target sequence over multiple cycles.
Advantages of Quantitative Real-Time PCR
Real-time PCR has a multitude of advantages over conventional PCR techniques, including greater sensitivity, higher specificity, and rapid result since the entire assay is completed in a single reaction. The method is also less time-consuming, less labor-intensive and also reduces the chance of contamination since there is no post-amplification procedure required.
TaqMan real-time PCR has the sensitivity to detect and quantify DNA or RNA targets present in very low abundance and because the probes are designed for a specific the target sequence, higher specificity is achieved, which minimizes the occurrence of false positive results compared to conventional PCR. However, a major challenge of the assay is in the design of the TaqMan primers and probes for a target sequence. It can be a complex and challenging task that requires a deep understanding of the target sequence and probe design principles.
We will discuss some common design pitfalls and how online resources such as the Genscript Real-time PCR (TaqMan) Primer and Probes Design Tool can help simplify the process and ensure success.
Challenges in Designing TaqMan Primers and Probes
One of the primary challenges with designing primers and probes for real-time PCR TaqMan is ensuring sequence specificity. The primers and probes must be designed to target the desired sequence with high specificity to avoid binding to non-target sequences, which could result in false positives or inaccurate measurements.
Another challenge is optimizing the primer and/or probe melting temperature (Tm) at which the primers anneal to the target sequence during PCR amplification. is not optimal for the real-time PCR reaction. If the annealing temperature is too low, non-specific binding can occur, and if the annealing temperature is too high, the primers may not bind efficiently, resulting in low amplification efficiency and low sensitivity.
Other parameters including the length and GC content of the primers and probes can affect their specificity and efficiency. Primers and probes that are too short or too long may not bind efficiently to the target sequence, while those with high GC content can lead to non-specific binding. Also, designing primers that generate a very long amplicon may lead to poor amplification efficiency.
Lastly, it’s important to consider the potential for probe degradation during PCR amplification. If the TaqMan probe is not stable, it may degrade and release fluorescent signal prematurely, leading to false positives. This can be mitigated by choosing a probe with stable fluorescent dyes or modifying the probe chemistry to increase stability.
GenScript Real-time PCR (TaqMan)Primer and Probes Tool
GenScript offers a user-friendly online bioinformatics tool to design highly specific and efficient primers and probes for real-time PCR assays, which is available for free on the GenScript website. It can automatically design TaqMan probes based on user-provided target sequences.
The tool utilizes advanced algorithms to design TaqMan primers and probes that accounts for several important parameters including specificity, melting temperature, GC content, self-complementarity, and cross-reactivity. Users can select the species of interest and input the desired target sequence and parameters such as amplicon size, Tm range, and exclusion regions, and the tool will generate optimized primers and probes that meet the specified criteria. The output of the GenScript Real-time PCR Primer & Probe Design Tool includes detailed information on the designed primers and probes, such as their sequences, melting temperatures, GC content, amplicon length, and the presence of hairpin loops or secondary structures to ensure the accuracy and specificity of the design.
Overall, the GenScript Real-time PCR Primer and Probe Design Tool is a powerful and user-friendly online tool that can help researchers design high-quality TaqMan primers and probes for real-time PCR experiments, saving time and valuable resources in the experimental process.
Access the online tool here: https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool