
GMP manufacturing of mRNA lipid nanoparticles – Challenges and Opportunities
The rapid onset of the COVID-19 pandemic challenged the global scientific community to find a way to combat the SARS-CoV-2 virus. Emerging from relative obscurity, the messenger RNA (mRNA) and lipid nanoparticles (LNP) technologies were pivotal to the rapid development of novel COVID-19 vaccines and since then they have been catapulted into the mainstream. mRNA is a modular technology defined by its great flexibility with respect to production and application. The mRNA backbone’s physicochemical characteristics remains the same even as the sequence changes based on the target antigen, allowing it to be easily adapted to new pathogens and be manufactured using standardized product-agnostic manufacturing platforms that can revolutionize future pandemic response capabilities across the globe.
Unsurprisingly, interest and investment into both mRNA and LNP technologies are at an all-time high and biopharma companies are looking to extend the potential of these genomic medicines beyond prophylactic vaccines for infectious diseases to breakthrough treatments for cancer, rare diseases and more. However, as a relatively new technology, there are barriers to overcome to achieve successful commercial production of LNP-encapsulated mRNA (mRNA–LNPs) drug products. Cost-effective and scalable manufacturing processes coupled with well-defined product characterization will be needed to meet the growing demand for mRNA vaccines and other RNA-based therapeutics.
A recently published article, Process & analytical insights for GMP manufacturing of mRNA lipid nanoparticles, Cell and Gene Therapy Insights, written by Pall scientists examines the key manufacturing steps and discuss challenges and opportunities associated with the process and manufacturing facilities to produce these novel drug products. The benefits of engaging with an end-to-end solutions provider who can lend their expertise and deliver adaptive strategies to support changing industry needs is also discussed.
Gene Delivery Strategies
Gene delivery vehicles known as vectors are responsible for introducing genetic material (i.e., DNA, RNA, oligonucleotides) into cells, broadly categorized into viral and non-viral vectors.
Viruses, modified to disable their replication capability, are used as viral vectors and several types can be used to deliver nucleic acids into the genome of target cells including retrovirus, lentivirus, adenovirus, and adeno-associated virus. However, as pathogens, viruses are still naturally immunogenic and can cause significant immune responses in patients, which not only reduce vector penetration and treatment efficacy, but increases the risk for health complications. While researchers have taken steps to reduce the immunogenicity of viral vectors (i.e., incorporating suicide genes), non-viral alternatives are being increasingly adopted.
Classical non-viral delivery methods are based on physical strategies (i.e., electroporation, ballistic delivery) that increase the cell membrane permeability to permit the entry of nucleic acids, but they are not suitable for in vivo gene delivery. Lipid nanoparticle (LNP) technology is emerging as a desirable delivery technology especially for RNA because the LNP encapsulates the mRNA within a protective shell to protect it from RNAse degradation enroute to the target cell. Being non-viral, the risk of genome integration is low and LNPs are demonstrating their safety and tolerability in real-time as COVID-19 vaccines continue to be administered to individuals globally with minimal side effects. Additionally, LNPs can encapsulate RNA that encodes for any protein antigen with minimal change to chemical characteristics. They can also package large genetic payloads and enable multiplex gene delivery where multiple payloads are packaged within the same formulation (i.e., Cas9 mRNA and sgRNA). This platform technology provides opportunities for lipid raw materials to be pre-purchased, while common equipment and analytical methods can be used to produce RNA drugs for different applications to ease the burden of multi-product manufacturing.
Process & GMP Manufacturing
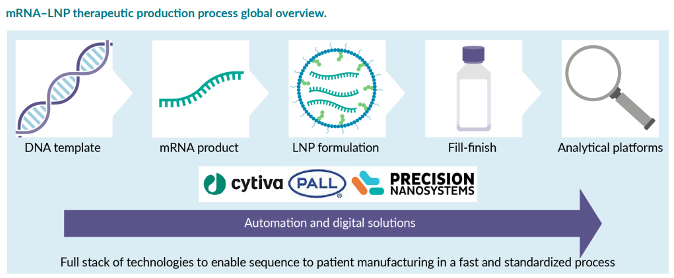
As a more nascent drug platform, there is no single, standardized manufacturing workflow, which puts the onus on manufacturers to develop and optimize their own process, leading considerable variation in the selection of processes and equipment both in the upstream and downstream unit operations. There are many variables and decisions throughout that will influence the equipment selection, setup, batch cost, and throughput. The authors of this article focus on a few of the more challenging and specialized steps across the workflow for mRNA-based therapeutics from in vitro transcription (IVT) to final drug product.
Capping Strategy. During IVT, one key process consideration is the mRNA capping strategy, which can be executed co-transcriptionally, or enzymatically as a separate reaction. There are trade-offs to each process, which can impact efficacy, timelines, and overall cost. For example, co-transcriptional capping is less expensive and faster than enzymatic capping since it occurs in the same reactor mix as the IVT. However, capping efficiency and yield are typically lower and non-capped impurities or cap analogs incorporated in the wrong orientation can be produced, which will need to be removed downstream. Whereas enzymatic capping has a very high capping efficiency, but it is more expensive and requires an extra unit operation, which can extend process time.
mRNA-LNP Formulation. The formulation method involves mixing lipids dissolved in an organic solvent with RNA in an acidic buffer to induce spontaneous self-assembly of mRNA–LNP particles. This process is governed by complex intermolecular interactions and requires fine control over the chemistry and the mixing environment to produce particles of the correct size and morphology, which are intricately tied to their biodistribution and in vivo function. Emerging technologies based on microfluidic mixing offer access to non-turbulent, well-controlled mixing environments that are reproducible batch-to-batch and readily scalable to support the industry as it looks to translate mRNA medicines beyond vaccines to more advanced therapies.
Formulation Considerations. Because of the shear sensitive nature of the mRNA-LNP intermediates, careful optimization of the final tangential flow filtration (TFF) formulation and sterile filtration downstream is required to mitigate any perturbations in their quality attributes such as size, shape, and polydispersity. Process optimization rooted in thorough process knowledge can mitigate risk and enables cost and time-efficient decisions.
Capital Equipment. As mRNA technologies continue to evolve, the key for capital expenditures will be to focus on flexibility. Modularized single-use equipment that can accommodate rapid configuration for a range of products at different scales can allow manufacturers to easily modify their facilities to meet evolving needs. Also, while not a requirement for process development, implementing cGMP-compliant single-use equipment early on could help simplify and expedite technology transfer and scale up to manufacturing for clinical use, which can ultimately drive products faster through the pipeline.
Filling Operations. It is common for drug manufacturers to outsource filling operations to external organizations. However, there can be a strategic benefit to have in-house platforms that are dedicated to advancing drug candidates through your own pipeline. The translucent nature of mRNA-LNP drug products presents additional challenges because visual quality checks aren’t possible, making them vulnerable to particulate contamination from conventional filling machines. Robotic filling systems capable of closed, aseptic operations mitigates this risk while also enhancing process control and offers flexibility for multi-product facilities.
Analytics
Regardless of how well-controlled the manufacturing process, it is crucial to analyze critical quality attributes (CQA) of the drug substance are maintained throughout. Intimate knowledge of CQAs and how critical process parameters (CPPs) can affect them can help inform process decisions at critical go-no-go junctures. As such, fit-for-purpose process analytical technologies (PAT) are required to provide crucial information with respect to process performance, stability, and product quality prior to batch release (i.e., identity, purity, potency, and safety.
Where there is a choice of analytical method, the merits of in-line, at-line, and off-line testing need to be balanced against the impact of the proposed assay on the process flow and length of time required for development of these methods. There are many distinct molecular intermediates across the manufacturing workflow including linearized plasmid DNA as starting material, purified mRNA as drug substance, and formulated mRNA–LNP as the drug product, all of which require analytical testing for in-process controls, product release, and stability programs. Examples of analytical methods for in-process testing, drug substance process (i.e., mRNA IVT and its purification) and the formulated bulk process (i.e., mRNA encapsulation and formulation) are discussed at length in the article.
The authors caution that given the relative novelty of the technologies and accelerated development timelines, we have limited experience with respect to their CMC, non-clinical, and clinical performances, product quality analysis and specification. Therefore, it is worthwhile to establish a comprehensive suite of analytical technologies and testing strategies that will aid the industry as a whole as more development programs come online.
Data Management
The relatively short processing time mRNA therapeutics could set the stage for manufacturing facilities to operate at a high throughput where many batches are produced on the manufacturing line per year. As a result, data management and batch release can become a bottleneck, regardless of the production scale. Investment into automated data management solutions can support efficient batch release and enable readiness for future production capacity. In particular, Manufacturing Execution System (MES) systems and centralized data repository (and off-site backup) are key technologies for GMP manufacturing to provide full audit trails for each batch run and on-demand access to process data that can streamline product manufacturing, testing, and final product release.
Facilities Considerations
The sensitivity of mRNAs to RNase contamination dictates that the mRNA manufacturing should be separated from other cell-based processes within a common facility. It could be worthwhile for a company to create a dedicated manufacturing environment for mRNA altogether to eliminate the risk depending on future products in the pipeline and anticipated manufacturing output. Modular prefabricated facility designs as a stand-alone operational unit or nested into existing facility space provide increase flexibility and new avenues to bring decentralized vaccine production to the point of need with speed, flexibility, and predictability.
As an important side note, the mRNA–LNP is formulated using a solvent injection technique (i.e., ethanol), so manufacturing facilities need to be designed to handle this specialized process as well as have the correct approvals to work with the volumes of reagent needed for the intended manufacturing scale. Be aware that different global regulations apply, so working with local authorities is essential to prevent any setbacks.
What’s Next
While the potential of mRNA-based therapeutics is huge, there is still much room for improvement and innovation. The authors look at several trends on the horizon as this modality continues to mature and evolve. Many of the technologies used early on were originally designed for other industries (i.e., therapeutic protein production) but as demand for mRNA-LNPs grows for vaccine development and other modalities, manufacturing technologies designed specifically for this modality to meet the production scale and maximize productivity are needed. It will take a collaborative effort between drug developers, solution providers and manufacturers to understand the emerging needs to find the best fit solutions moving forward.
Driving down the cost of production is also cited as an area to de-risk future manufacturing. IVT is currently the most cost-driving step in the mRNA process, therefore alternatives to batch production could help increase the overall process productivity and reduce the usage of costly reagents. The pDNA template for IVT is also used in viral vector applications, another growing area of biologics, which has strained the already limited supply. One possible solution would be to use a cell-free technology, such as rolling circle amplification, to generate the DNA template faster and easier.
LNPs represent another area of focus. Further development and optimization to identify formulations that offer better thermostability to overcome the need for cryostorage and engineering tissue specificity to improve mRNA drug delivery to specific targets can support their broader application across a variety of therapeutic areas. Developing the LNPs systematically, by screening libraries and optimizing parameters, and then modeling the full unit operation at small scale can facilitate these goals.
Drug developers are investing in innovative partnerships to improve segments of their workflow. For instance, some leading mRNA companies are working with artificial intelligence (AI) companies to employ rational design approaches often used in biomolecular engineering to mRNA sequence design, bringing new insights and opportunities. Ongoing collaborations with machine learning and cloud providers, like the one between Moderna and Amazon Web Services (AWS), are enabling faster experimental design, automation capabilities and data integration to simplify technology transfer and meet regulatory compliance.
From a regulatory perspective, the industry would benefit from globally harmonized standards, yet these criteria have yet to be fully defined. In the meantime, future mRNA–LNP development should leverage the experience gained from the accepted regulatory framework established by the approved COVID-19 vaccines to accelerate regulatory approvals across different countries and agencies.
The relative newness of the mRNA-LNP technology means there is still uncertainty surrounding the best approaches to process development and manufacturing with the best process economics. Because of the rapidly changing landscape of available technologies and tools, there is tremendous value to developers to connect with end-to-end solution providers who can provide evolving and tailored strategies to support changing industry needs. As manufacturing challenges are resolved, we will truly see influence of mRNA technology in the future trajectory of vaccine development, oncology, and personalized medicine.
To download a copy of the full article, please visit: Process & analytical insights for GMP manufacturing of mRNA lipid nanoparticles