
GMP Proteins for Cell Therapy Manufacturing: Top 6 Things to Know
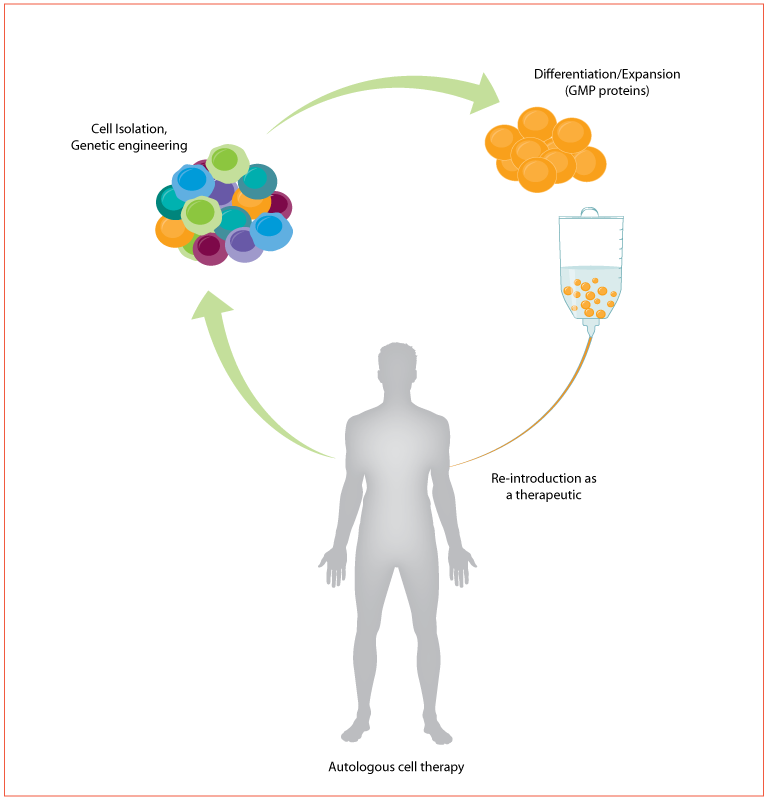
Using cells as a therapeutic offers one of the most promising new approaches for treating human disease. These methods include stem cell therapies and regenerative medicine, as well as immune cell therapies for cancers and immune-related disorders. Despite the promise, it has been difficult to bring these therapies mainstream. They are vastly more complex than traditional small molecule treatments or biotherapeutics. For example, in autologous therapies like CAR T, cells are removed from the patient, enriched for the cell of interest, genetically engineered to target the tumor, expanded in cell culture, and re-introduced into the patient as the therapeutic (Figure 1). Many of these steps have yet to be standardized and, along with the inherent variability in biological systems, there is increased potential for unanticipated results and risk to patients.
One commonality across virtually all cell therapies is the use of cytokines and growth factors for the differentiation, expansion, and maintenance of cells. These proteins themselves are made in biological systems meaning that they are susceptible to variability. Consequently, Cell Therapy developers and manufacturers typically choose proteins that a supplier classifies as “GMP”, which refers to good manufacturing practices. Traditionally the use of GMP has pertained to compliance to specific regulations applied to FDA-regulated products. However, in this context there is no requirement for oversight by a regulatory body. These materials are designed for therapeutic manufacturing and are removed before direct contact with the patient. Other examples that might fall into this class are magnetic beads, serum, and culture media. The “GMP” classification for manufacturing purposes is typically used to refer to the quality system that is in place to ensure that products are consistently produced and controlled according to standards designed to minimize variability and associated risk.
There remains well-founded confusion about the definition of GMP and what a supplier of GMP proteins for manufacturing should be able to provide. Below we’ve listed 6 key characteristics to look for.
Top 6 things to know when selecting a GMP protein manufacturer
1. Expect Suppliers to be Knowledgeable and Meet Pertinent Requirements
Two published sources of generally accepted requirements are available: Unites States Pharmacopeia (USP) Section <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products, and the recently published (2017-01-01) European Pharmacopeia (Ph. Eur.) General Chapter 5.2.12 Raw Materials of Biological Origin for the Production of Cell-based and Gene Therapy Medicinal Products. These publications can provide a good starting point to review in preparation for identifying and selecting a suitable protein supplier. However, they are guidelines and definitions can vary among protein manufacturers. The responsibility ultimately falls on the developer of the Cell Therapy to determine whether a given supplier meets their needs.
2. Consistency from Lot-to-Lot
Consistency may be the most obvious of the characteristics to look for when choosing GMP-level proteins for clinical manufacturing. During an on-site audit, a vendor should be able to show you data from a minimum of 3 lots to establish that they can reproducibly manufacture a protein. This means consistency in characteristics such as activity and purity, as well acceptable endotoxin levels. This might be critical for managing the scale-up of a future therapeutic, where multiple lots may be needed to fulfill the needs of larger patient populations. Some proteins are more difficult to produce than others, and a vendor should be able to make you comfortable with their abilities. That said, you should obtain samples from multiple lots to ensure acceptable results for use in your specific analytical testing and Cell Therapy culture system.
3. Ability to Scale
There is much discussion surrounding the difficulty in moving cell therapies from clinical trials into commercial production. Some of this struggle revolves around a lack of standardized equipment designed for meeting the needs of large patient pools or managing logistics associated with the location of the patient vs. the labs where cells are grown and manipulated. Raw materials like serum and growth factors can also be expensive. It is also important to note that some cytokines and growth factors are easier to scale than others. Developers should have a good idea of what their future needs might be at scale to ensure that they choose a supplier that offers the best combination of consistency at scale and price. Early discussions about quality agreements and supply agreements will help avoid surprises as you progress to production. You have to be convinced that a manufacturer can meet your future needs in order to avoid late-stage changes that might require costly revalidation.
4. Secondary Suppliers
Secondary suppliers should be identified early. The same growth factor or cytokine sourced from different vendors may look identical on a certificate of analysis, but could behave differently in your cell culture system. You should never assume that you can easily switch between vendors for a given protein. Always have a backup plan.
5. Experience Counts
Experience in protein manufacturing can add value to the relationship between you and your supplier. As mentioned earlier, the responsibility for raw material qualification resides with the developer or manufacturer of the Cell Therapy. An experienced protein manufacturer will be in a position to share details about the biochemistry of a given protein and assist you in determining its suitability for your system. If the vendor has proprietary information, drug master files/master files should be available that can be referenced in regulatory submissions. A well-qualified vendor may even suggest adjustments to the manufacturing process to create a protein that will better meet your needs. A supplier with experience in navigating the changing regulatory landscape can also add value. It’s fair to inquire about the vendor’s experience with your protein of interest, as well as their experience in the Cell Therapy space. You should expect more than just a vendor-customer relationship. It should be a partnership.
6. Quality Management Systems
At minimum, a vendor should have an ISO 9001:2008/2015-certified quality management system (QMS). This means the supplier has been independently audited and has policies and processes in place designed to meet the needs of their customers. At the core of the QMS is extensive document and record management system that includes written procedures, comprehensive manufacturing records, manufacturing SOPs, a supplier qualification monitoring program, equipment calibrations and maintenance records, and identification/traceability of materials starting from incoming inspection to finished product release. Also included are ways to deal with non-conformance and to make improvements to existing processes. Some vendors may also be ISO 13485:2003/2016 certified. This certification pertains to materials which may be classified as medical devices, or may be used in the production of medical devices. In the case of growth factors and cytokines, the ultimate goal of the QMS is to ensure the production of consistent reagents that decrease risk to both manufacturer and patient.
R&D Systems manufactures the largest selection of GMP proteins on the market.
- Click here to read more about their GMP capabilities and quality manufacturing guidelines.
- Click here to view the full GMP protein portfolio.