
Hassle-free nanoparticle characterization with Stunner
The use of nanoparticles in medicine offers exciting new possibilities to deliver RNA, DNA, and protein payloads with more diverse therapeutic applications than ever before. Notably, lipid nanoparticle carriers are integral to the success of the COVID-19 messenger RNA (mRNA) vaccine aimed at activating immune cells against the SARS-CoV-2 virus. The diversity of potential payloads and applications has led to the creation of many forms of organic nanoparticles and includes liposomes, lipid nanoparticles (LNP), solid lipid nanoparticles, nano-emulsions, polymeric lipid nanocarriers and other proprietary or specially formulated particles for targeted delivery.
The ability to execute rapid and accurate nanoparticle characterization was the focus of a recent webinar presented by Nelis Denys of Unchained Labs. As Nelis described, once a nanoparticle platform is produced, there are two key questions developers will need to characterize: nanoparticle size and payload concentration.
Stunner platform by Unchained Labs is uniquely designed to be able to address these two attributes simultaneously with its UV/Vis and dynamic light scattering (DLS) technologies. UV/Vis absorbance determines the total payload concentration while DLS is used for nanoparticle sizing. Nanoparticle sizing is particularly important, with numerous publications identifying a correlation between nanoparticle potency (effective dose) and size.
How does Stunner Work?
Stunner utilizes a specialized high-throughput 96-well microwell plate requiring just 2 µL sample/well with built-in microcuvettes at two fixed pathlengths (0.1mm and 0.7mm). After the sample plate is loaded into the instrument, the empty circuit is read first to compensate for any absorbance contribution from the plate itself. Vacuum pressure then moves the sample into the microcuvettes to be read. The specific nanoparticle applications on the machine can execute total payload quantification (whether it is RNA, DNA, protein, or another active pharmaceutical ingredient), and acquire size and polydispersity index (PDI) of nanoparticles simultaneously from both UV/Vis and DLS technologies.
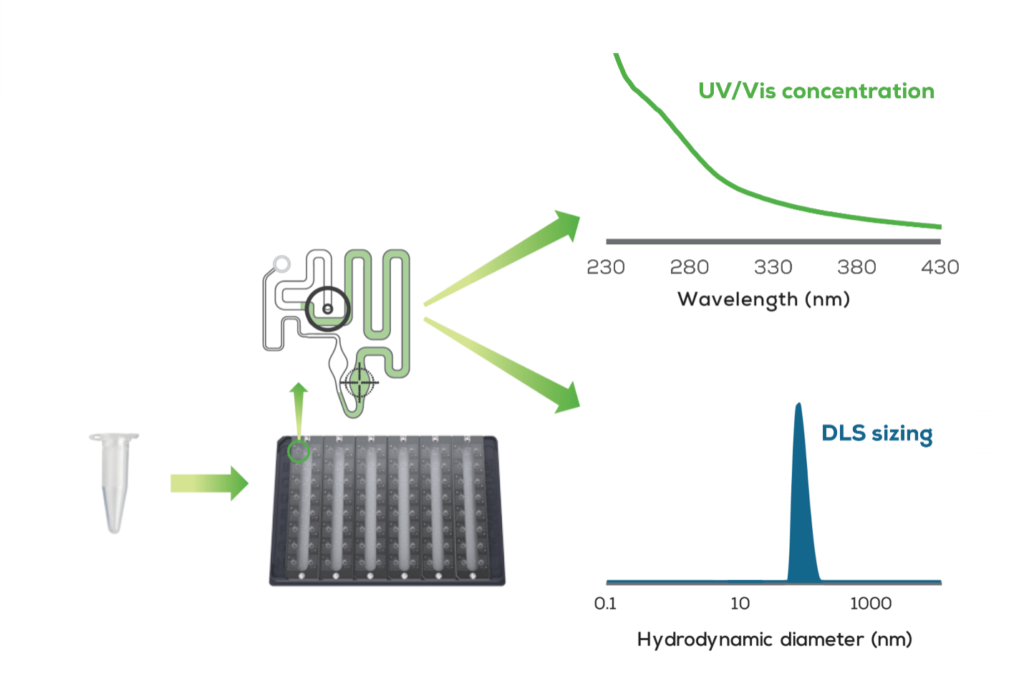
Nanoparticle Sizing
Stunner’s DLS requires only 2 µL sample volumes and the high-throughput 96-well plate format provides rapid and accurate nanoparticle size and PDI data to accelerate workflows, which can be critical when analyzing high numbers of samples. Stunner offers additional advantages over other DLS systems by reducing hands-on time (10mins vs. 3hrs) and turnaround time, where 96 samples can be run in less than one hour.
Payload Concentration
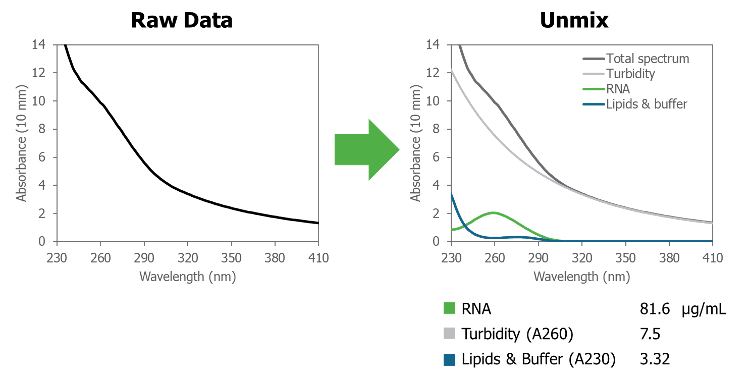
Typically, payload quantification (i.e., RNA, DNA, proteins) can be easily executed with traditional UV/Vis. However, samples containing LNPs carriers tend to have high turbidity that conventional methods can’t overcome. The empty LNPs create an absorbance signal alongside that of the payload that confounds quantification. Therefore, it is necessary to isolate the absorbance signal from the RNA from the LNP turbidity, UV-absorbing lipids, excipients, and buffer components.
The Unmix algorithm can effectively isolate the impact of turbidity and remove it from the absorbance spectrum of the sample, as well as resolving the signal contribution of other components. As shown below, the RNA absorbance can be clearly visualized once the Unmix algorithm has removed the interference from the turbidity.
How does Stunner compare?
Concentration data obtained in a direct comparison study between Stunner and RiboGreen RNA quantification was comparable and a 2-fold dilution series of Firefly luciferase-mRNA-LNPs showed highly linear results down to 1.2 µg/mL (error bars are +/- 1 standard deviation). Added benefits over RiboGreen include quantification without the need for standard curves, dyes, and complex sample handling, which limits throughput and adds to the expense for RiboGreen workflows.
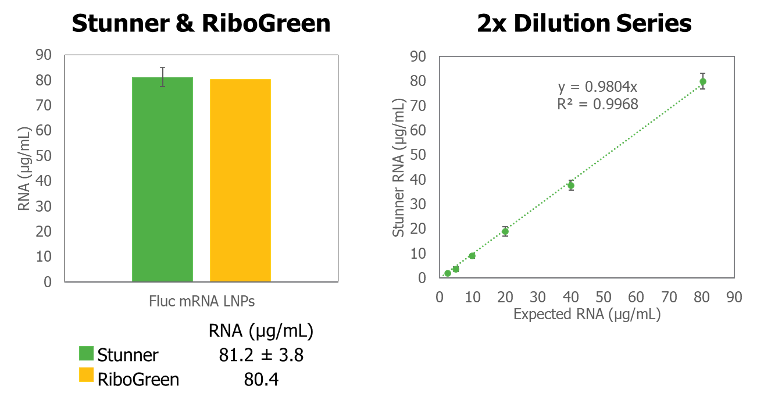
Data from Stunner also demonstrates reproducibility, with little variation observed across 96 RNA-LNP replicates, with a calculated coefficient of variation of 1.54%.
As Nelis noted, while the data presented features RNA-LNPs, other payloads like DNA- and protein-LNPs measured on Stunner produce accurate and precise data that is linear across its complete range.
Customization Available
Another feature of Stunner and nanoparticle applications is it can be customized to your nanoparticle and payload formulation. A diversity of new, unique and proprietary particles with their custom payload are being developed rapidly. The software provides the ability to set up a custom reference spectra by running the pure payload sample, which the software can deconvolute from everything else.

As Nelis described, there are IQ/OQ procedures available, and Stunner software is 21CFRp11 compliant with full audit trail and electronic signature capabilities. To seamlessly integrate into manufacturing and quality control workflows, Performance Verification tests that adhere to United States Pharmacopoeia and European Pharmacopoeia guidelines for UV/Vis spectrometers are available.