
Increase Protein Yield by Striking the Right Balance between Cell Density and Protein Productivity
A Guest Blog by Stacey Garcia, Ph.D., Product Manager, Essential Pharmaceuticals
For decades, bioprocessing engineers have spent significant resources on optimization to improve recombinant antibody production. The first clones developed had low productivity (in the mg/L range), but since that time, significant advances in process development have been made in cell productivity and protein quality to achieve titers of 3 g/L and higher (1). Chinese hamster ovary (CHO) cells are commonly used for antibody production for a variety of reasons, including their capability for rapid growth in suspension and high protein productivity. Bioprocessing engineers continue to optimize cell lines (including CHO cells) and media and feed strategies to further improve the efficiency of cell production and the efficacy of the protein products.
Yield is determined by how much protein is secreted per cell and how many total cells are secreting protein. This formula has lead to several different approaches to increasing protein yield. One approach to increase protein yield is to increase the total number of cells. In order to increase the number of cells, large bioreactors up to 25,000 liters would be used. A second approach is to increase the number of cells in the same volume, effectively increasing viable cell density. This approach has been taken to the limits in single-use systems, where viable cell density (VCD) is very high. The third approach to increase protein yield is to increase the amount of protein made per cell, called protein productivity. Often a mix of these strategies are employed to increase protein yield.
When one looks at increasing VCD to increase protein yield, VCD is defined as the number of cells that are alive in a given volume of media. Regardless of the approach to increase protein yield, VCD is commonly measured during upstream process development. Several different screening methodologies are available to scientists to measure performance improvements while optimizing a media strategy for a particular CHO cell line. One popular screening tool is to track the VCD of different conditions to determine proliferation rates and viability of the cells for the length of the run. Ultimately, a combination of measurements, for example VCD and protein titer, would be analyzed to determine which media strategy would be chosen for the clone.
How do proliferation rate and VCD relate to protein titer, if at all? In this blog, we examine the relationship between cell growth and protein productivity with the help of a study evaluating cell metabolism in fed-batch CHO cell culture.
To Grow or Produce? Different Cell Metabolism is Required for Different Functions
A typical growth curve for cells in a production run begins with early exponential growth and is followed by late exponential growth, stationary phase, and decline (cell death). Both cell metabolism and protein productivity can vary through each stage of the production run. In a study performed by a group at Amgen, antibody production and cell metabolism were evaluated at each fed-batch phase using 13C labeling and metabolic flux analysis.
Antibody production was found to be at its minimum during early exponential phase and at its highest during stationary phase. As nutrient availability and cell density changed over the course of the production run, cell metabolism also changed. For example, cells took in the most glutamine and produced the most lactate in early exponential phase, while they took in other amino acids and produced the most carbon dioxide during stationary phase. In general, peak specific growth rate was associated with high lactate production, and peak antibody production was associated with high oxidative pentose phosphate pathway activity. Their findings confirmed that cell growth and protein production are not correlated and have very different nutrient uptake and metabolism (2).
What do these findings say about the relationship between VCD and protein productivity? The study shows the relationship between declining biomass (or less total cell density) and an increase in monoclonal antibody. The late stage of the cell lifecycle is when the CHO cell is a highly productive protein factory. Associated with those different stages of the life cycle are different resource needs observed over the bioproduction run. In effect, active proliferation and increasing cell density is counter to protein productivity. Therefore, fast proliferation and increased VCD may not correlate with an increased protein yield. For example, a shake flask with high protein productivity per cell may lead to the highest overall protein titer, even if it has less total cells than the control shake flask. In this case, the VCD may not correlate with protein titer.
Of course, there are situations where VCD and protein titer do correlate. As an example, a correlation might be observed when a cell population is growing quickly and producing protein, leading to a high cell count and high overall protein titer. However, a high number of cells producing protein is not always desirable, as there may be issues downstream with purification when starting with a high biomass.
For bioprocessing engineers looking to maximize protein yield, it is critical to achieve a balance between number of cells and protein productivity. A production run must include a high enough amount of cells with high protein productivity to have the desired result. It can be a challenge to optimize a system so that a balance is achieved.
Conclusions and Solutions
When the goal is to increase protein yield, VCD is a valuable metric to gauge the proliferative capacity of the cell, but it is not the only indicator of protein yield. Experimental conditions (such as media or feeds) that increase per cell productivity may have equal or lower VCD than the control, while still increasing overall protein yield. Therefore, it is valuable to measure total protein yield and per cell productivity alongside viable cell density.
Cell-Ess® universal protein titer boost and optimizer was developed by Essential Pharmaceuticals, LLC to improve efficiency by increasing protein productivity without increasing biomass. Cell-Ess is chemically-defined, animal component-free and produced under cGMP conditions. In recent studies, Cell-Ess used as an initial supplement at 1% (v/v) in a serum-free system increased monoclonal antibody in raw titer by 25% and on a per cell basis by 40%. In addition, Cell-Ess used as a feed in a fed-batch model increased titer by greater than 30% compared to the protein titer when Cell-Ess was added only as an initial supplement. The increase in protein titer was observed in multiple media systems, indicating that the addition of Cell-Ess as a tool for media optimization is not dependent on the current media system being utilized.
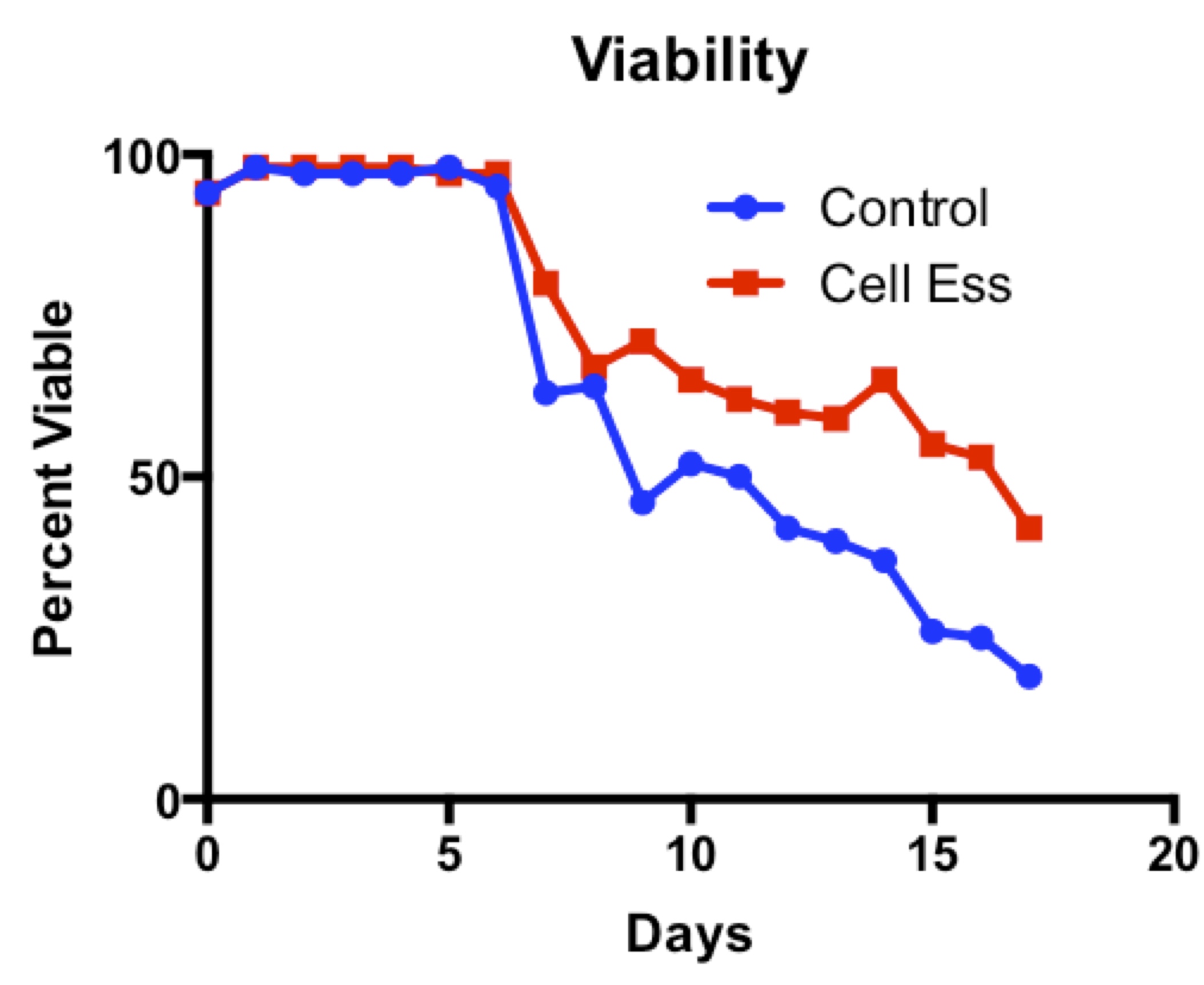
Cell-Ess has been shown to increase protein titer without increasing proliferation, which is consistent with the findings of the Amgen study discussed here. In addition, Cell-Ess has been shown to maintain cell viability for longer than control, enabling scientists to extend the length of the bioreactor run to generate more protein, if desired. As the Amgen study has shown that stationary phase is correlated with high protein productivity, a supplement or feed that improves viability during stationary phase may result in improved protein productivity.
The ability to extend the bioproduction run due to improved cell viability could provide additional operational efficiencies, for example if one less bioreactor run can be performed in a year to produce the same amount of protein. By increasing protein productivity without increasing biomass, Cell-Ess may help reduce issues downstream during purification.
Cell-Ess is a tool to help bioprocessing engineers find the balance between number of cells and protein productivity. Achieving a balance between the number of cells and protein productivity ensures that the bioproduction system reaches its maximum protein yield. Essential Pharmaceuticals has evaluated the impact of Cell-Ess on protein productivity in a variety of cell lines, media systems, and manufacturing conditions. Click here to download recent case studies outlining our findings.
References
1Rader RA and Langer ES. Biopharmaceutical Manufacturing: Historical and Future Trends in Titers, Yields, and Efficiency in Commercial-Scale Bioprocessing. Bioprocessing Journal 2015 13:4, 47-54.
2Templeton N, Dean J, Reddy P, Young JD. Peak Antibody Production is Associated With Increased Oxidative Metabolism in an Industrially Relevant Fed-Batch CHO Cell Culture. Biotechnol Bioeng. 2013. 110(7): 2013-2024.