Innovative Chemicals for Process Intensification in Cell Culture Media
A Guest Blog by Ronja Seibel, Caroline Hecklau, Alisa Schnellbaecher, Maria Wehsling, and Aline Zimmer
Process guidance for the use of the modified amino acids Phospho-Tyrosine Disodium Salt EMPROVE® EXPERT and Sulfo-Cysteine Sodium Salt Sesquihydrate EMPROVE® EXPERT in fed-batch processes.
Introduction
L-Tyrosine is a key amino acid for both cellular metabolism and protein synthesis and its depletion in fed-batch processes has been correlated with a drop in specific productivity1 and with protein sequence variants.2 This critical amino acid presents an extremely low solubility, especially at neutral pH.3,4 The use of tyrosine di-sodium salt concentrations above 1 g/L in feeds induces precipitation and increases the risk of media instability, mainly through co-precipitation of other amino acids. This may lead to sub-optimal performance due to insufficient supply of nutrients and finally to low performing processes.
L-cysteine is a sulphur-containing amino acid which is oxidized to the dimer L-cystine in the presence of air, oxygen or metal containing catalysts such as copper.5 L-cysteine is freely soluble, L-cystine has a reduced solubility in water6 and often precipitates in neutral pH feeds.
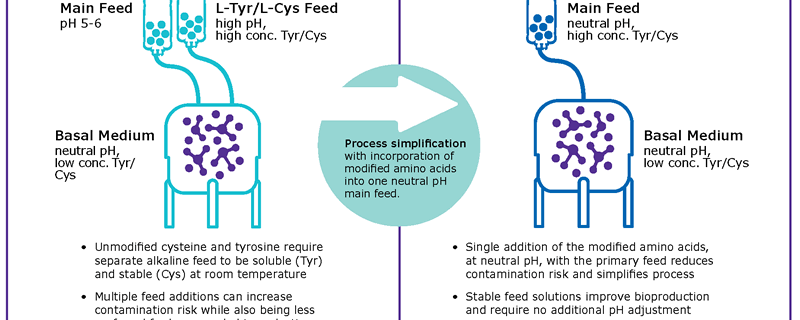
To overcome these limitations, common fed-batch processes use highly concentrated alkaline feeds which create the need for complex control strategies to minimize pH spikes during feed additions (Figure 1A). In order to remove this alkaline feed, tyrosine and cysteine were chemically modified to phospho-L- tyrosine disodium salt7 and S-sulfocysteine sodium salt8 respectively. The combination of both chemicals allows the integration of both amino acid sources in highly concentrated, neutral pH feeds (Figure 1B) and thus provide a unique way to simplify fed-batch processes by enabling the development of stable and neutral pH main feeds.
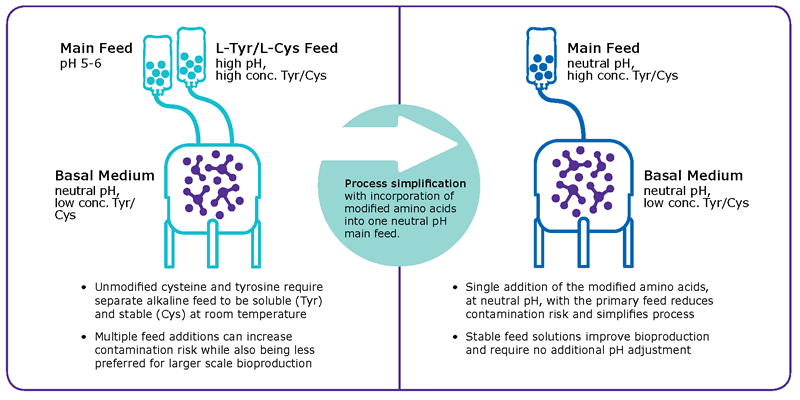
Performance
Solubility and stability
In water, the solubility of phospho-L-tyrosine disodium salt (PTyr) was evaluated at 53 g/L, more than 100-fold higher than the solubility of L-tyrosine (0.38 g/L). In concentrated feeds, PTyr2Na+ exhibited a solubility of 70 g/L in contrast to L-tyrosine, which was not soluble and to L-tyrosine disodium salt for which the maximum solubility was < 1 g/L.
No degradation or precipitation of phospho-tyrosine was observed during the first six months in liquid feed, and no free tyrosine generated from potential oxidation reactions was detected. S-Sulfocysteine sodium salt (SSC) was soluble up to 1.3 M in water at room temperature and 50 mM SSC were soluble in the pH 7.0 feed. In water, SSC was stable at acidic and neutral pH, whereas a spontaneous dissociation leading to cysteine release and cystine formation was observed at alkaline pH.
There was no visible precipitation or change in color during three months storage in neutral pH feed at 15 mM SSC. There was no significant decrease of the SSC concentration in the feed stored at 4 °C or room temperature protected from light.
SSC is known to interact with other thiol-containing molecules like cysteine, glutathione or monothioglycerol. To ensure SSC stability, we recommend removing any thiol containing components from target feed formulations.
Performance in Fed-batch processes
The performance of both modified amino acids was tested in fed-batch using several CHO cell clones producing different IgG1s.8,9 In all processes tested so far, equimolar concentrations of both cysteine and tyrosine proved to be the best concentrations for both PTyr and SSC in the feed. However, specific cell line requirements may require a titration experiment for both amino acids independently.
In addition, our studies highlighted the essential role of the tyrosine concentration in the medium used in the fed-batch process.7 Since PTyr is cleaved in the cell culture media by cellular phosphatases, the kinetic of Tyr release may vary between cell lines. Therefore, we recommend monitoring the tyrosine content in the spent medium during the entire fed-batch. Since a minimum of 1 mM tyrosine seems to be required to ensure efficient transport of the amino acid into cells,2 we recommend to adjust the tyrosine concentration in the medium in order to exceed that concentration at any time during the process. In the process used in our studies, this resulted in an adjustment of the tyrosine concentration in the medium from 1 mM to 2.5 mM.
Finally, in our hands, phospho-tyrosine cannot be used as replacement of tyrosine in the medium. For sulfo- cysteine, the replacement of cysteine in the medium lead to different results depending on the clone. Our recommendation is to avoid modifying the cysteine concentration in the medium.
Results obtained in a standard Fed-batch process using a CHO cell line producing an IgG1 are presented in Figure 2.
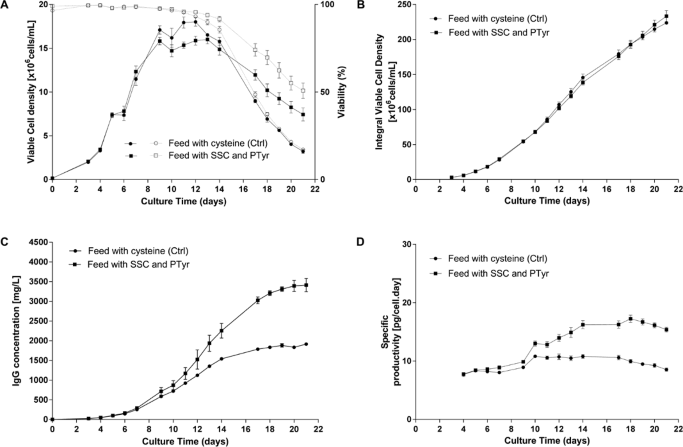
(B) Integral viable cell density, (C) IgG concentration in the supernatant measured by a turbidometric method, (D) Specific productivity.
Impact on the monoclonal antibody
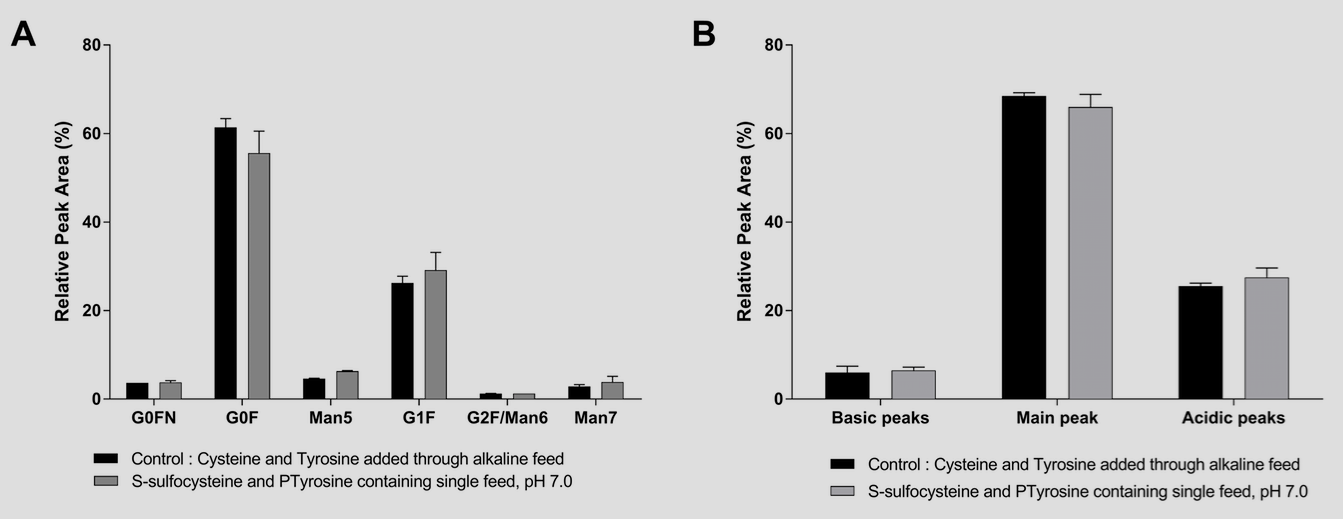
The use of PTyr and SSC in concentrated feeds did not result in a detectable modification in the amino acid sequence7,8 or the glycosylation or charge variant profile of a model IgG1 (Figure 3 A and B).
In depth analysis of the antibody allowed us to demonstrate a reduction in the IgG heterogeneity when SSC was used in the process.9 Indeed, the use of this modified amino acid resulted in a reduced fragmentation of several IgGs as well as in a reduced level of trisulfide bond linkages between the heavy chain and the light chain of the mAb (Figure 4).
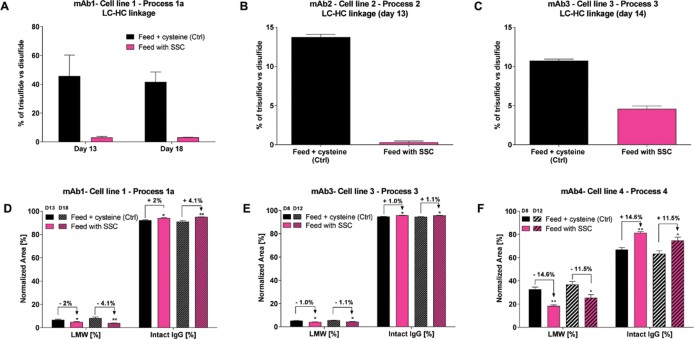
A to C: Trisulfide bonds between light chain and heavy chain of fours IgGs were compared with the disulfide linkages and quantified using LC-MS. D to F: Fragments were separated according to their size under non-reduced conditions using CE-SDS. Statistical differences were assessed by Kruskal-Wallis and Dunn’s multiple comparison tests. p-values of less than 0.05 (*) and 0.01 (**) were considered significant.
Benefits
Modified amino acids allow development of neutral pH, highly concentrated and stable cell culture media formulations
- Easily soluble tyrosine derivative Phospho-Tyrosine
- Stable cysteine derivative Sulfo-Cysteine enabling
- Reduction of recombinant protein fragmentation
- Decrease in trisulfide bond content
Simplified process
- Fewer feed stream preparations
- Easier process configuration
- Less contamination risks
- Prevention of local caustic shocks due to high pH
Reduce costs
- Save preparation time and efforts for various feed containers
Efficient process
- Reduced volume to be processed in upstream and downstream
Reliable supply
- Large manufacturing capacities ensure reliable supply for our customers
Emprove® Program
We market Sulfo-Cysteine sodium salt as well as Phospho-Tyrosine di-sodium salt as part of our Emprove® Program, which combines outstanding quality with comprehensive documentation and excellent service.
Each product in the portfolio is complemented with three types of dossiers to help facilitate your qualification, risk assessment and process optimization efforts: Material Qualification Dossier, Quality Management Dossier and Operational Excellence Dossier. They provide information on the manufacturing process, stability data, elemental impurity information, product quality reports, analytical procedures, and much more. The Emprove® Program includes 400 pharma raw and starting materials and a selection of filtration and single-use products.
The dossiers can be accessed online in our Emprove® Suite. All our subscribers to the Emprove® Suite get 24/7 access to all dossiers of the entire Emprove® portfolio for two years, with up to five accounts per company. The Material Qualification Dossier continues to be available free of charge at EMDMillipore.com.
For more information about the Emprove® Program, visit EMDMillipore.com/emprove or ask your local sales representative.
Specifications
The full product specification can be found at: SigmaAldrich.com
Safety Data Sheet
The current safety data sheets can be retrieved from the website: SigmaAldrich.com
Footnotes
-
1. Yu, M., et al., Understanding the intracellular effect of enhanced nutrient feeding toward high titer antibody production process. Biotechnol Bioeng, 2011. 108(5): p. 1078-88.
-
2. Feeney, L., et al., Eliminating tyrosine sequence variants in CHO cell lines producing recombinant monoclonal antibodies. Biotechnol Bioeng, 2013. 110(4): p. 1087-97.
-
3. Carta, R. and G. Tola, Solubilities of l-Cystine, l-Tyrosine, l-Leucine, and Glycine in Aqueous Solutions at Various pHs and NaCl Concentrations. Journal of Chemical & Engineering Data, 1996. 41(3): p. 414-417.
-
4. Hitchcock, D.I., The Solubility of Tyrosine in Acid and in Alkali. J Gen Physiol, 1924. 6(6): p. 747-57.
-
5. Rigo, A., et al., Interaction of copper with cysteine: stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation. J Inorg Biochem, 2004. 98(9): p. 1495-501.
-
6. O'Neil, M.J., et al., The Merck Index. 2006, Merck Research Laboratories, Merck & Co., Inc.: Whitehouse Station, NJ, USA. p. 467-468.
-
7. Zimmer, A., et al., Improvement and simplification of fed-batch bioprocesses with a highly soluble phosphotyrosine sodium salt. J Biotechnol, 2014. 186: p. 110-118.
-
8. Hecklau, C., et al., S-Sulfocysteine simplifies fed-batch processes and increases the CHO specific productivity via anti-oxidant activity. J Biotechnol, 2016. 218: p. 53-63.
-
9. Seibel, R., et al., Impact of S-sulfocysteine on fragments and trisulfide bond linkages in monoclonal antibodies. MAbs, 2017. 9(6): p. 889-897.