Isolation of Mononuclear and Polymorphonuclear Leukocytes from Blood with Density Separation Media
Blood is a specialized body fluid composed of four main components: plasma, white blood cells (leukocytes), red blood cells (erythrocytes), and platelets (thrombocytes). Unlike red blood cells and platelets, all white blood cells are nucleated and can be classified by their nuclei structure as mononuclear or polymorphonuclear cells. White blood cells have an important role in immunity to protect the body against both infectious disease and foreign invaders cementing their utility for immunological studies. Thus, isolation of mononuclear and polymorphonuclear cells from blood serves as the starting point for a wide spectrum of immunology studies. Lymphocytes and monocytes are the two main categories of mononuclear white blood cells with one-lobed nucleus in the immune system with specific and important roles in defending the body from infection, cancer, and other foreign invaders. Lymphocytes include natural killer (NK) cells, T (thymus) cells, and B (bone marrow- or bursa-derived) cells and are the main type of cell found in lymphatic system. In humans, lymphocytes make up the majority of the peripheral blood mononuclear cell (PBMC) population (comprising 70-90% of the PBMC population), followed by monocytes (10-30% of PBMC population), and a small percentage of dendritic cells (1-2% of PBMC population). PBMCs are important for immunological studies and clinical applications such as diagnostics and disease treatment. Some key applications of PBMCs include immune response assessment, biomarker identification, vaccine development and cancer immunotherapy. Early methods for isolating leukocytes involved mixing blood with a compound which aggregated the erythrocytes and to a much lesser extent, the leukocytes. With centrifugation, these aggregated erythrocytes pelleted due to their increase density, while the leukocytes remained in solution. However, the recovery of leukocytes was less than desired because the cells can be trapped by and sedimented with the aggregated erythrocytes. In 1968, BØyum1 introduced a more convenient and rapid separation using centrifugation through a Ficoll-sodium metrizoate solution. This separation method takes advantage of cell density differences of the components in whole blood that, when centrifuged in the presence of a density gradient media, exhibits a unique migration pattern through the medium allowing distinct cell populations to be fractionated. Distinguished from mononuclear leukocytes, polymorphonuclear leukocytes have the varying shapes of the nucleus, which is usually lobed into three segments. They are responsible for combating bacterial and fungal infections. Neutrophils are the most abundant polymorphonuclear leukocytes, and the other types (eosinophils, basophils, and mast cells) have much lower populations. A pain point for many researchers is how to specifically isolate mononuclear and polymorphonuclear cells from blood with high yield and cell viability. MP Biomedicals offers three products for the isolation of mononuclear and polymorphonuclear cells from human peripheral blood as well as bone marrow, and umbilical cord blood. The lymphocyte separation medium (LSM™), LymphoSep®, and Mono-Poly® Resolving Medium have been used for many applications by researchers worldwide.
1. Mononuclear Cell Isolation for Research Use
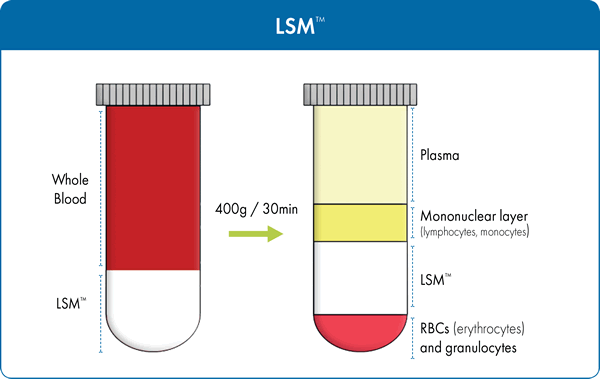
Modifications of the BØyum formulation have been made by numerous researchers. The Lymphocyte Separation Medium (LSM™) produced by MP Biomedicals, has a unique formulation where sodium diatrizoate is successfully substituted for sodium metrizoate. LSM™ allows for the separation of lymphocytes not only from human peripheral blood, but also from bone marrow as well as umbilical cord blood. It has a density of 1.077-1.08 g/mL consisting of 6.2g Ficoll and 9.4g sodium diatrizoate per 100mL of solution. Figure 1 summarizes the separation principle when using LSM™ to isolate lymphocytes from the other blood constituents. This time tested and proven separation medium has over 2,200 scientific publications and was designed to ensure:
- Maximum yield of mononuclear cells
- Easy one-step centrifugation
- >96% cell viability of lymphocytes
- Low endotoxin
- Autoclave sterilization
Examples of Applications using LSM™
Mononuclear cell isolation from human peripheral blood Liu et al2 isolated monocytes from the peripheral blood of axial spondyloarthritis (AxSpA) patients and healthy controls to study differential tumor necrosis factor alpha-induced protein 3 (TNFAIP3) regulation in blood-derived macrophages. This study demonstrated that monocytes isolated with LSM™ retain their differentiation capabilities and can produce functional M-CSF-derived macrophages for use in immunological studies. The resulting macrophages from AxSpA patients were found to have decreased levels of the anti-inflammatory protein TNFAIP3 indicating that it is a potential contributor to cytokine dysregulation observed in this disease. Lymphocyte preparation from mouse and human tumor cells Seo et al.3 successfully used LSM™ to isolate lymphocytes from both human and mouse MHC class I-deficient tumor cells. The isolated lymphocytes were utilized to determine the percentage of tumor infiltrating NK cells within these tumors after recombinant interleukin 21 (rIL21) was administered. The researchers were able to show that intratumoral delivery of rIL21 attracted NK cells to the tumor site. Mononuclear cell isolation from rat spleen cells LSM™ has also been used successfully to isolate lymphocytes from rodent species even though they have a slightly higher average density than in humans. In a recent study4, the paracrine effects of mesenchymal stem cell (MSC)-derived exosomes, after acute myocardial infarction, on angiogenesis and anti-inflammatory activity was examined. In this case, rat spleen lymphocytes were isolated using LSM™ to perform T-cell proliferation assays used to assess the angiogenic potency of MSC-derived exosomes. Isolation of parasites from feces or blood LSM™ has been demonstrated to be effective in isolating parasites from peripheral blood samples. Lymphatic filariasis is a tropical, mosquito vector-borne disease caused by filarial nematodes, such as Brugia malayi. Microfilariae (Mf) or first-stage larvae are ingested by mosquitoes that can infect humans through mosquito bites. Schoeder et al5 successfully utilized LSM ™ to isolate the microfilariae (Mf) from the blood of infected gerbils. The Mf was co-cultured with HUVEC cells (isolated from human umbilical cord) to study the mechanism of Mf sequestration in the lungs of infected individuals.
2. Lymphocyte Separation for in vitro Diagnostics
LymphoSep® lymphocyte separation medium is based on the original BØyum formulation, which was originally designed for the in vitro isolation of lymphocytes from diluted whole blood. It has a density of 1.077 g/mL. LymphoSep® is validated for In Vitro Diagnostic (IVD) usage and has designation as an FDA class I exempt medical device for lymphocyte separation (21CFR864.8500). Similar to Lymphocyte Separation Medium (LSM™), LymphoSep® provides:
- High yield of lymphocyte cells
- >96% cell viability of lymphocytes
- Easy one-step centrifugation
- Low endotoxin
- Autoclave sterilization
Defibrinated or heparinized blood specimens are first diluted with physiological saline or balanced salt solution in 1:1 proportion, layered over the separation medium, and centrifuged at a low speed for 30 minutes. During centrifugation, differential migration of blood constituents results in the formation of several cell layers. The following layers will be visible in the conical tube, similar to that observed with LSM™ (Figure 1), from top to bottom: blood plasma and other constituents, a layer of mononuclear cells called buffy coat (PBMC/MNC), separation medium followed by a pellet at the very bottom which contains granulocytes erythrocytes (red blood cells). To isolate PBMCs, the buffy coat is extracted, washed with salt-buffered solution, and then centrifuged allowing the cells to be recovered with high yield in a small volume. The supernatant, containing platelets, separation medium, and plasma, is removed, leaving a pellet of purified PBMCs. These cells can then be used in clinical and scientific applications and investigations.
Examples of Applications using LymphoSep®
Mononuclear cell isolation from umbilical cord blood A recent study was conducted to better understand the prenatal origin of pediatric B-cell acute lymphoblastic leukemia (ALL). MNCs isolated from umbilical cord blood (using LymphoSep™) from a large cohort of newborns were screened for the presence of the most common and prognostically important pre-leukemic fusion genes (PFG), formed in embryonic/fetal development, of B-lineage ALL6. RT-qPCR was performed on the MNC to establish levels of PFGs such as ETV6-RUNX1, MLL-AF4 and BCR-ABL1 (p190). Mononuclear cell isolation from peripheral blood Martin and Whalen7 endeavored to study exposure to environmental toxicants pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT) and how they affect the secretion of interleukin 1-beta (IL-1β) in human immune cells. The effect of the toxins was evaluated using various immune cell preparations such as human NK cells, human monocyte-depleted (MD) peripheral blood mononuclear cells (PBMCs) (MD-PBMCs), and PBMCs. White blood cells obtained from leukocyte filters were layered onto LymphoSep™ separation medium to separate the PBMCs from the granulocytes. The MD-PBMCs were obtained by incubating PBMCs in glass Petri dishes for 1 hour to allow the monocytes to adhere to the dish, effectively depleting them from the suspension.
3. Polymorphonuclear Cell Isolation
Often times, researchers are not only interested in isolating the mononuclear cell population. When it is necessary to separate both mononuclear and polymorphonuclear cells from blood, Mono-Poly™ Resolving Medium (Mono-Poly™, M-PRM) may be used. It is specifically designed for optimal separation of mononuclear lymphocyte, polymorphonuclear leukocytes from contaminating erythrocytes in a one-step centrifugation. M-PRM is a solution composed of a polysaccharide (Ficoll 400) and a radiopaque contrast medium (Hypaque) in a specific ratio to yield a density of 1.114± 0.002 g/mL. Undiluted, anti-coagulant-treated blood is layered over M-PRM prior to centrifugation (Figure 2). Differential migration during centrifugation allows for the resolution of both mononuclear and polymorphonuclear leukocytes into two distinct bands that are relatively free of erythrocytes.
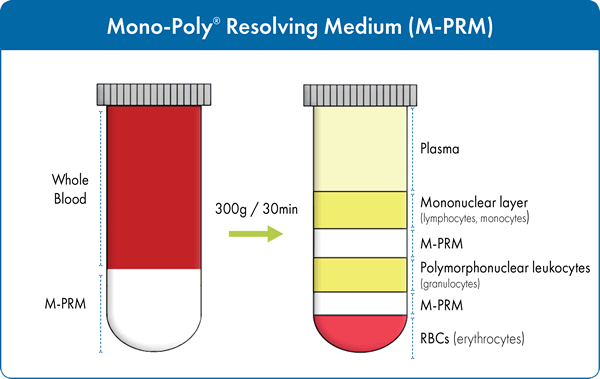
Typical results from various laboratories have shown that separation of blood on M-PRM can achieve >90% leukocyte recovery with >99% viability as assessed by the trypan blue dye exclusion method. Generally, the top leukocyte band (Fraction 1) contains 94-98% mononuclear cells (lymphocytes and macrophages) while the second (Fraction 2) contains 96-99% polymorphonuclear leukocytes. Both the mononuclear and polymorphonuclear leukocytes isolated by this system have been shown to retain functional properties. In particular, the polymorphonuclear cells fractionated using M-PRM have better functional capacities than those prepared by other methods. For example, M-PRM has been used for the isolation of neutrophils8,9,10,11 from blood for use in a wide variety of assays for immunological research. These phagocytic granulocytes are the first line of defense against infection acting by ingesting and/or releasing enzymes that kill the microorganisms. They are an essential part of the innate immune system. The isolation of mononuclear and polymorphonuclear cells from blood, bone marrow and cord blood can be used in numerous assays to provide valuable insights into health and disease. Individual subpopulations, such as CD8+ T cells which are important cells in immuno-oncology, can be further isolated for more specific studies. Therefore, it is desirable to obtain the purest, most functionally viable cells at the highest yield possible from your specimen of choice. For more information on any of the MP Biomedical products discussed here, please visit https://www.mpbio.com/immuno.php/isolate
Footnotes
-
1. Bøyum, A. Isolation of mononuclear cells and granulocytes from human blood. (Paper IV). Scand. J., Clin. Lab. Invest. 21 Suppl, 97 (1968): 77–89.
-
2. Liu, Y et al. “Genetic and Functional Associations with Decreased Anti-Inflammatory Tumor Necrosis Factor Alpha Induced Protein 3 in Macrophages from Subjects with Axial Spondyloarthritis.” Front Immunol. 8 (2017): 860.
-
3. Seo, H et al. "IL21 Therapy Combined with PD-1 and Tim-3 Blockade Provides Enhanced NK Cell Antitumor Activity against MHC Class I–Deficient Tumors." Cancer Immunol. Res. (2018). 17-0708.
-
4. Teng, X et al. “Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation.” Cell Physiol. Biochem. 37 (2015):2415-24. doi: 10.1159/000438594.
-
5. Schoeder JH et al.” Brugia malayi microfilariae adhere to human vascular endothelial cells in a C3-dependent manner”. PLoS Negl. Trop. Dis. 11(2017): e0005592.
-
6. Kosik, P et al. "Low numbers of pre-leukemic fusion genes are frequently present in umbilical cord blood without affecting DNA damage response." Oncotarget 8 (2017): 35824.
-
7. Martin, TJ., and MM Whalen. "Exposures to the environmental toxicants pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT) modify secretion of interleukin 1-beta (IL-1β) from human immune cells." Arch. Toxicol.91 (2017): 1795-1808.
-
8. Francischetti, IMB et al.” Evidence for a lectin specific for sulfated glycans in the salivary gland of the malaria vector, Anopheles gambiae.” PloS One, 9(2014), e107295.
-
9. Chagas, AC et al. “Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma.” PLoS Pathogens, 10(2014): e1003923.
-
10. Su, HW, et al. “Rapid dielectrophoretic characterization of single cells using the dielectrophoretic spring.” Lab on a Chip, 13 (2013): 4109–17.
-
11. Sakamoto, H., et al. "Macromolecular Activators of Phagocytosis from Platelets (MAPPs) and Their Activator Peptide, HATKTAK." Ann. Pharmacol. Pharm. 2 (2017):1080.