
Laboratory based therapeutic and diagnostic marker protein concentration through centrifugal and crossflow ultrafiltration
Ultrafiltration is an important tool in concentrating therapeutic and diagnostic marker proteins. Two of the primary methods are using centrifugation or crossflow ultrafiltration. When considering the ultrafiltration method that is best for your application, it is critical that you select the appropriate method for target recovery and sample volume, while still considering speed and efficiency.
Efficacy of ultrafiltration processes can be very application dependent and thus may raise technical questions in process planning.
To answer these questions, this week we are excited to have Rik McRae, Technical Manager for Operations, Sartorius Stedim Lab, Ltd. as our expert. Rick has over 20 years of experience in product and application research and development, production engineering and technical guidance and is currently a member of the Sartorius lab ultrafiltration team. Rik is a subject matter expert for technical support and application knowledge, with strong expertise in therapeutic protein, drug delivery nanoparticle, and diagnostic marker concentration applications, with centrifugal, pressure driven and lab scale tangential flow filtration systems.
Question 1
I’m having problems concentrating a large volume sample. I’ve been centrifuging but it takes over two hours and I’m losing a good portion of my protein. Do you have any recommendations for a better way?
Yes, concentrating medium to high volume samples can be time-consuming and costly in the laboratory setting. Happily, there are some good solutions for concentrating up to 5L of sample in the lab setting, without having to resort to the high priced processing scale crossflow systems that require large mounting system and high capex investment. Below provides a rough outline of the technologies for lab-based ultrafiltration at the different volume ranges.
100uL to 500uL: Vivaspin 500 centrifugal concentrator (fixed-angled rotors accepting 2.2ml centrifuge tubes)
400uL to 2mL: Vivaspin 2 centrifugal concentrator (swing-bucket or fixed-angled rotors accepting 15ml falcon tubes)
2mL to 6mL: Vivaspin Turbo 4 or Vivaspin 6 (swing-bucket or fixed-angled rotors accepting 15ml falcon tubes)
4mL to 20mL: Vivaspin 15R, Vivaspin Turbo 15 to Vivaspin 20 (swing-bucket or fixed-angled rotors accepting 50ml falcon tubes)
20mL to 100mL: Vivacell 100 (most centrifuge buckets that fit 250ml bottles)
50mL to 5L: Vivaflow 50, 50R or Vivaflow 200 (cross flow / tangential flow filtration cassettes, only a 200-400ml/min speed peristaltic pump needed)
Depending upon the specific volume, it is likely either the Vivacell 100 or one of the plug and play Vivaflow TFF systems will enable rapid concentration of large volume samples. After concentrating to your desired concentration factor a low volume flush can be put through the Vivaflow cassettes to maximize recovery. Typical concentration times are; 250mL to <20ml in several minutes, ~1L to a concentration factor of 50 in <30 minutes and 5L in <75 minutes.
Question 2
We are looking for the best method for concentrating protein in cell culture media while not degrading the protein.
There are two main causes for protein degradation during ultrafiltration processes; unbalanced protein to pH/sample buffer conditions and high shear stresses. Certainly the more linear the protein shape the greater the adverse effects on structure caused by high g-forces. If the protein in question is linear we would recommend having a slower concentration period with lower g-forces than the maximum recommended (~50% the recommended is a good starting place). For more standard globular molecules it is good practice to use a device closer to the minimum volume, this ensures a higher surface area and reduces the potential for blockage and increasing stress on the samples. Although it should be noted that with higher surface areas it increases the rate of non-specific binding if you have a non-optimal protein-membrane combination or if your protein is considered to be “sticky”. Although the advent of vertical membranes took away most of the issues of blockages caused by dead-end filtration. Laboratory-based TFF cassettes take that one step further and offer a true parallel flow path for the sample, further ensuring minimal shear stress. It should be noted that typically stirred cell vessels put on relatively high amounts of shear stress.
For pH/buffer conditions, once the correct buffer ratio is selected it can be maintained using continuous buffer exchange techniques (diafiltration). These are offered both with the Vivaflow devices and the centrifugal Vivaspin 20 and ensure that even with increasing concentrations the correct buffer balance is maintained, or a new buffer added if needed.
One final point to ensure the best concentration method is to test and consider the ultrafiltration membrane used, as it’s not always a one-stop-shop. So test out what is best, usually either RC, PES or CTA (CTA is more commonly used when interested in the filtrate, as it has a lower internal non-specific binding).
Question 3
When would you recommend cassettes or centrifuge?
When we’re talking about lab research applications this largely comes down to what your sample volume is. The Vivaflow cassettes that operate by tangential flow filtration (TFF) for example provide the optimal recovery and speeds of concentration from the 100mL to 5L range. Less than 100mL and you find centrifugation options generally provide the best recoveries. Cost efficiency applies to this also, as it’s a lower device cost and time cost by using a single Vivaflow 50 cassette for 150ml than 10x Vivaspin Turbo 15 devices for example.
However, there are method-specific attributes that apply for both. For example, TFF devices can perform concentration at a consistent transmembrane pressure (TMP), whereas centrifugal concentrators tend to start with a high TMP then decrease as the process goes on. A changing TMP can have an impact on a particularly sensitive proteins stability and means there are more variable shear forces. However, happily the Sartorius Vivacell 100 that works with a 20-100ml volume range can be pressurized as well as centrifuged. When pressurized a far more consistent TMP is applied and so these are well suited for the concentration of protein complexes due to the low and regulated shear forces. Further to this TFF devices can be used for general clarification of the filtrate techniques.
Centrifugal devices on the other hand often come in a wider range of style and MWCOs, stretching from 2K MWCO with our patented Hydrosart membrane, to our PES 1,000K MWCO and 0.2um membrane types. Further to this, there are a number of centrifugal devices that are designed for specific applications, such as the Vivacon range that are designed for DNA concentrations, the Centrisart range which is optimal for filtrate recovery and Vivaspin Endotest which allows for the complete retention of endotoxins.
Finally, centrifugation is generally considered easier as most have a centrifuges around the lab and most crossflow devices are manufactured for the bioprocessing industry and so require high capex and fiddly mounting systems. Although happily that’s not the case anymore with the plug in and play Vivaflow devices, that just require a peristaltic pump.
In summary, it depends on your sample volume, specific application and protein sensitivity.
Question 4
What MWCO and membrane is best suited for viral vector concentration, such as Lentivirus or AAV?
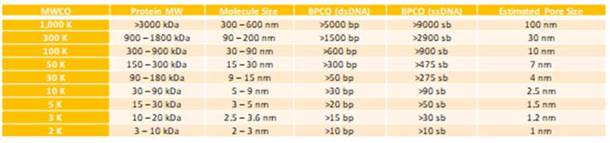
We’re hearing a lot more about viral vectors and vaccine applications with the growing trend in regenerative medicine, so this is an area we’ll be doing more dedicated research on in the near future. However in general the principles of membrane and MWCO selection are the same as with proteins and other biomolecules, unless there are specific considerations for your virus. The rule of thumb is to choose a molecular weight cut off (MWCO) close to one third the molecular weight of your target. In the case of viruses where molecular weight is not as relevant as diameter there is a comparison chart which gives the best MWCO for diameter and DNA length. For Lentivirus for example which has a ~90nm diameter we find that 300K MWCO membranes work optimally, with 100K MWCOs also giving good results at the sacrifice of speed.
The below link provides a review of ultrafiltration protocols for virus concentration and purification.
Question 5
What are the key considerations in maximising protein recovery from centrifugal concentrators?
Good question, this is a process that should always be considered as there are so many different types of proteins out there and the same membrane, MWCO, device, etc is not always best for each. The following steps should be taken in considering how best to improve concentrations and recoveries; 1) consider the sample and molecule properties, changes to pH can increase conformational rearrangements, lower temperatures can reduce concentration rates, etc. 2) Pick the right membrane, UF is known for not having many membrane options, but you should at least test whether regenerated cellulose (RC) or Polyethersulfone (PES) is best for your protein and use the right membrane for each protein. 3) Select the right MWCO and device, typically a MWCO 1/3 the size of the target is suitable and a device that is right for the total sample volume. 4) Use appropriate device treatment methods, such as pre-rinsing to remove analytes or flushing with a non-interfering protein to passivate the binding sites and reduce loss. Finally, 5) Consider sample control methods, such as using dead stops within devices to pipette out all the retentate or pre-filling the filtrate tube to control the final volume of the retentate.
Question 6
Can ultrafiltration work with non-biological targets such as polymers?
Absolutely, with increasing drug discovery research requests on polymers are becoming more common. Ultrafiltration devices can used for both concentration and washing steps. A short review at the below link gives some examples. https://promotions.sartorius.com/fileadmin/knowledgedatabase/Filtration/Appl_Guide_Nanoparticles_SL-4001-e.pdf
Question 7
Can raw cell culture be placed through cross flow ultrafiltration? What’s the best way to minimise blockages with culture supernatant?
This doesn’t often happen as blockages are pretty likely with raw cell culture straight from the bioreactor, particularly with the higher cell densities. You could try it with a TFF devices as these are the best for minimising risk from blockages, but even still it’s a long shot. If you need to use an ultrafiltration style set up it may be a better bet to use a 0.2um TFF device for clarification before going on to the concentration step with a MWCO ultrafilter. Alternatively you could use more common clarification steps such as by using diatomaceous earth based filtration devices such as Sartoclear Dynamics Lab filter range, standard GF or PP depth filter capsules or a simple centrifugation step with low volume samples.
Question 8
For buffer exchange of proteins that are very sensitive to pH and concentration changes, e.g. those that precipitate out easily, what’s the best way to handle this?
Diafiltration! General dialysis can work well too, but it takes a long time, often overnight, and often leads to sample loss handling delicate dialysis bags. Whereas with diafiltration using ultrafilters you can perform the steps independently, but also in parallel to an ultrafiltration step. I.e. you can add a buffer reservoir to a lab based TFF system, that will draw in fresh buffer as the sample concentrates. There are even centrifugal concentrators that can house a buffer cup that allows for diafiltration whilst in the centrifuge, such as in the following link.
https://www.sartoriusglobal.com/mediafile/Appl_Vivaspin-20_Diafiltration-cups_SL-4076-e.pdf
Question 9
When using AAV vectors have you used ultrafiltration for virus stocks and if so what do you recommend and how does it compare to traditional methods?
Yes AAV vectors stocks are often concentrated and polished using ultrafiltration and this generally works well. Commonly we seen ultrafiltration as being best used after a microfiltration step (clarification of larger cell debris) and prior to another purification step such as column or membrane chromatography (to remove similar sized HCP molecules, etc). In this workflow the ultrafiltration step aids in a further clarification by removing particles that are 10x larger or 10x smaller than the chosen MWCO*, whilst also concentrating the virus stock to a more workable concentration. However, ultrafiltration is also used in vector workflows for buffer exchange steps prior to chromatography and final polishing steps after chromatography.
In terms of recommendations it depends upon what is best for your specific process and end goals, happily we have a regenerative medicine team that can provide proposals specific to you should you be interested in more info. However in my opinion ultrafiltration with a 50K MWCO used in conjunction with a membrane chromatography step tends to be the most efficient for producing stock quickly whilst maintaining titres. Whereas methods such as microfluidization can have higher shear stresses and may need more optimisation, although I’m not an expert in these areas.
*Ultrafiltration can be used for separation of molecules based upon size, but due to the biochemical variables involved in ultrafiltration the rule of thumb is that true fractionation can only occur between molecules that have a 10 10x difference in molecular weight or diameter.
Question 10
What do you recommend for exosome isolation?
I’m happy to see this questions, as with the rise of drug delivery research we’re opting to invest more application research into the exosome and extracellular vesicle workflows, so hopefully soon we’ll be able to provide some application material in the future.
Once clarified from the cell culture using depth, micro or diatomaceous earth filtration (or a combination of them depending upon the density and viability or your culture or lysate), I would first identify or make an estimate on the size distribution of your exosome diameter range. The guide provided in previous questions shows what ultrafiltration MWCO is best suited for what diameter. To capture all exosomes we would recommend picking a MWCO relevant to the smallest likely diameter of the exosomes. So with a likely range of 20 – 200nm, we would recommend using a MWCO of 50K, which can capture particles as low as 15nm. This will ensure that all exosomes, regardless of size are retained. Whilst still allowing small contaminating molecules to pass through the membrane, further purifying the sample as well as concentrating. It may be necessary to carry out a size exclusion chromatography step to further purify the exosomes, as by using a MWCO low enough to capture all the exosomes you may capture a larger amount of proteins above the MWCO also. With this in mind, you can always select a MWCO based upon the targeted exosomes size also, not just the minimum possible.
Question 11
Have you looked at continuous ultrafiltration? What are your thoughts about best practices?
Within the lab products and services ultrafiltration technologies that we focus on we have not looked at this in too much depth. Primarily because lab applications tend to revolve around low volume sample batches that need one-off, single pass through runs. Where more than a single pass through is needed some devices can be used more than once (although rarely recommended!) or there is tangential flow filtration that allows for recirculation until concentration target is met. True continuous ultrafiltration is mainly applicable to the clinical dialysis market and more recently the bioprocessing market. The former is not our field of expertise, however, our Sartorius Bioprocess division is working on continuous filtration options for the biopharma industry. From their perspective it’s important to understand what is continuous TFF, per sample batch? Per consumable? Or one system running with self-cleaning and checking water flow after each process run? The ambr crossflow system has this for example, but with single use membrane cartridges only. As otherwise the problem of membrane fouling and how to remove this in process arises. Happily I understand they’re working on new technologies in this area and so may be interested in more in-depth discussion on this.
https://www.sartorius.com/en/products/process-filtration-purification
Question 12
We are looking for an ultrafiltration solution for our microbioreactor studies. What is the max sample volume you recommend with your systems?
It depends upon what method of ultrafiltration is best suited, however below are the typical volume ranges for our lab based upon the different types of force.
Centrifugation: 100ul to 100ml
Pressurisation: 20ml to 100ml
Tangential Flow Filtration: 100ml to 5000ml
So for example the Sartorius ambr 15 bioreactors produce working culture of 10-15ml, this would work directly with our Vivaspin Turbo 15 or Vivaspin 20 devices. Perhaps the Vivaspin Turbo 4 or Vivaspin 6 devices if some volume is lost after initial clarification.
Question 13
I need to make up MSC medium and I am looking to concentrate the proteins and let small molecules flow through.
The first action is to identify the expected sizes/molecular weights of the proteins you want to capture. From this you should choose an ultrafiltration devices that has a MWCO close to 1/3 the size of the protein. E.g. for a 100kDa protein choose a 30K MWCO, for a 50kDa protein try using a 10K MWCO. Like with the last example, if you have to pick a MWCO closest to 1/3 the size of the target, choose the lower option. As otherwise the membrane may be too open and let some target protein through. Small molecules will then pass through whilst the protein is retained. However it’s important to note that in picking a 30K membrane, it does not mean that 100% of molecules that are <30kDa will pass through. A good proportion of 25kDa proteins will be capture, less so for 20kDa and so on. For accurate separation with ultrafiltration there must be a 10x size difference between the molecules. In this instance you could be reasonably sure that most molecules <3kDa in size will pass through.
Question 14
Have you seen problems with membrane fouling? Do you have ways to prevent this.
This was certainly a hot topic a number of years ago when dead end ultrafiltration was still a common technique. Sartorius therefore were the first inventors of the vertical and dual vertical membranes used in most centrifugal ultrafiltration devices today. These vertical membranes provided a near parallel flow path across a membrane, meaning there was significantly less fouling and blockages. However if membrane fouling is still being experienced a few suggestions are; implementing of a new or more efficient clarification step, perhaps with a combination of diatomaceous earth and a 0.2um PES membrane filter. Or ultrafiltration through a true cross flow / tangential flow filtration (TFF) device, such as the Vivaflow range for lab use. If membrane fouling is being experienced even with TFF devices and a sturdy clarification process, and as a result a decline in flux is still being seen, it may be worth checking that if a multiuse membrane, the cleaning and storage procedures are being followed correctly. Perhaps using single use devices could resolve this. You could further investigate the membrane properties and test a new membrane to see if the hydrophilicity, charge, binding strength to contaminants, etc, could be having an impact. The two common UF membranes are PES or RC and both can work very well with most samples, but occasionally one is better than the other for a specific sample as ultrafiltration is impacted by both physical and chemical interactions.
Question 15
What membrane and would you recommend for purifying an 85 kDa protein?
Typically we would look to use a molecular weight cut off (MWCO) close to 1/3 the size of the target protein. In this case that would make a MWCO of ~28k, the closest membrane to this is a 30k which would be therefore be the suggested membrane. In other circumstances when in between possible MWCOs, we might suggest to use the lower MWCO to ensure that maximum recovery is reached, however in this instance the difference from 28k to 30k is negligible and so 30k is a good choice. Some may recommend 1/6 the size or ½ the size of the target, however with the former you risk too slow concentration speeds and with the latter you risk losing some protein to the filtrate or membrane matrix. The other consideration is what type of membrane to use for your protein, e.g. PES, RC, Hydrosart or CTA. This largely depends on your protein properties, however both PES and RC are good with most proteins. If you wish to confirm which is best we would suggest testing with sample devices.