Laminin cell culture matrices – The key to efficient derivation and reliable culture of stem cells and specialized cells lies within these extracellular matrix proteins
Ever since the derivation of the first line of human embryonic stem cells, researchers in areas of regenerative medicine, basic biomedical research, and drug discovery have all been striving towards more reliable and reproducible procedures to culture human pluripotent stem cells (hPSCs) and/or differentiated cells. Years of development have resulted in more defined culture methods, and although major media improvements have been made, undefined culture substrates are still being used and few are natural and cell type specific.
Reliable derivation and expansion of high quality hPSCs under feeder-free, defined and xeno-free conditions, rapid scale-up, efficient and reproducible differentiation and maintenance – all these areas are important for a Stem Cell Researcher, but many struggle to achieve it. Furthermore, for therapeutic applications, all these steps must comply with clinical requirements. Mimicking the natural cell environment is the key for success and the culture substrates play a vital role. Here, I will discuss the use of biologically relevant, human, recombinant laminin cell culture matrices from BioLamina, and how they can be used to recreate the cell-specific, physiological microenvironment on a cell culture dish. We call it biorelevance. Let me show you how laminins can help pave the way towards successful Cell Therapy.
Laminin biology
Most organized cells of the body grow on specialized extracellular matrices (ECM) called basement membranes, and different cells and tissues require their specific ECM composition for survival and proper function. Laminins (>16 different isoforms) are the main cell-type specific proteins of the ECM and are both spatially and temporally regulated. Cells bind to laminins via cell-surface receptors, such as integrins, which regulate vital cellular responses, like cell-anchorage, survival, proliferation and differentiation. Laminins are complex proteins and BioLamina is the only company that can supply different full-length recombinant laminin isoforms with their biological activities intact. Our chemically defined and xeno-free laminin cell culture substrates correctly imitate the natural cell-matrix interaction in vitro, and can be applied for many different applications, such as expansion of pluripotent cells and differentiation and maintenance of specialized cell types.
The laminin-521 stem cell matrix provides efficient derivation and robust and reliable expansion of pluripotent stem cells
The expression of laminin chains can be observed already in four-cell-stage embryos and in the blastocyst, laminins containing an α5 chain, such as laminin-521 and laminin-511, are abundant around the pluripotent human embryonic stem (ES) cells of the inner cell mass.
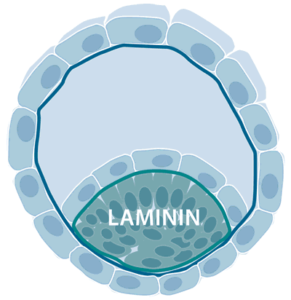
The α5 chain–containing laminins also surround many adult stem cells, such as cells in intestinal villus crypts, muscle progenitors and hair follicles. The prominent location of laminins in the stem cell niches suggests that they are of importance for the growth of stem cells in vivo. In 2014, Rodin et al. first described an efficient xeno-free and chemically defined protocol for monolayer culturing of hPSC on laminin-521 (Rodin et al., Nature protocols., 2014). Via specific binding of cellular receptors on hPSCs, laminin-521 triggers authentic cell signaling pathways that allows high survival and fast expansion of single-cell cultured hPSC, without the addition of ROCK inhibitor or any other inhibitors of anoikis. hPSCs in single-cell suspension, plated on laminin-521 rapidly attach, migrate and form small monolayer colonies. The cells rapidly divide and expand until the entire dish is covered by a confluent monolayer. hPSC cultured in thick multilayer colonies often recuire manual, arbitrary removal of undifferentiated parts. Contrary, hPSCs cultured
on laminin-521 grow as a homogenous monolayer, with minimal risk of spontaneous differentiation or genetic abnormalities. Moreover, the low batch-to-batch variation of the laminin substrates gives more consistent and reproducible experiments. The biorelevant culture environment generates hPSC cultures with a more uniform gene expression and gives a priming effect that enhances cell maturation, polarization and functional organization in many cell applications. The laminin-521 matrix also allows efficient clonal derivation, clonal survival and long-term self-renewal of human ES cells. Human ES cell lines can even be derived from a single blastomere under chemically defined and xeno-free condition on LN-521 (Rodin et al., Nature Communications, 2014), this without destroying the embryo and thereby circumventing the ethical issues associated with human ES cells. Laminin-521 allows for flexible and user-friendly culture protocols where the cells can be seeded at low densities (>5,000 cells/cm2) and be cultured to high (up to 99%) confluence. In addition, the laminin-521 culture substrate is compatible with any medium, any dissociation agent (e.g. enzymes and EDTA) and support weekend-free feeding.
Read more about the laminin-521 matrix
Biology is key to proper use of hES and iPS cells, paving the way towards successful cell therapies
The stem Cell Therapy field is still in its infancy and there are currently no GMP-grade substrates available that give reliable differentiation performance. Cell Therapy companies are still relying on undefined and non-human matrices. However, more and more focus is now invested on cell culture products that better relate to a future clinical translation. Due to the validated functionality and superior biological properties, human recombinant laminins in conjunction with streamlined differentiation protocols, offers exciting prospects for regenerative medicine. Laminin substrates have significantly advanced the Stem Cell Research field and have become a preferred substrate, both within basic research, as well as for researches and companies with a therapeutic focus. This has been highlighted in a number of high-impact scientific articles in the past two years and a few examples are described below:
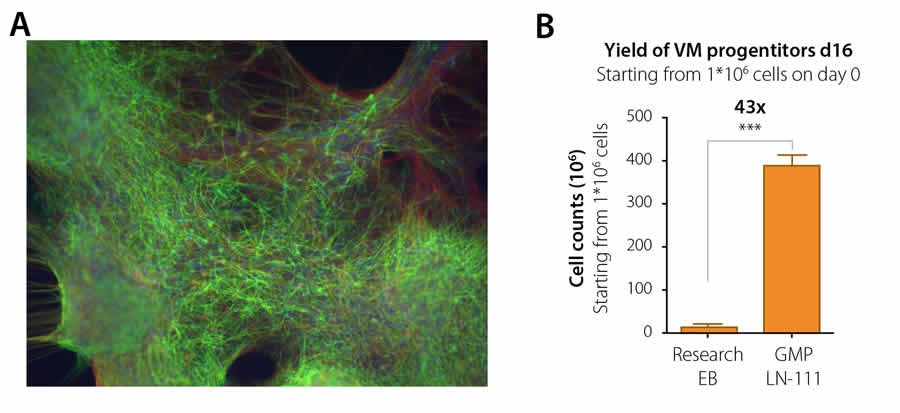
High yield of clinically compliant dopaminergic neurons
Current treatment regimes of Parkinson’s disease are based mainly on medicines that mimic dopaminergic signaling in the brain. These dopamine agonists and precursors initially work well but are limited by the development of loss of function along with increased side effects during treatment. Transplantation of dopaminergic neurons is a possible, major advance in the treatment of this decease. The group of Dr. Malin Parmar at the University of Lund, Sweden, recently published a groundbreaking article in Cell Stem Cell where they present a fully defined and GMP compatible procedure for dopaminergic cell differentiation of human ES cell lines on BioLamina laminin-111 (Kirkeby et al, Cell Stem Cell, 2016). Their protocol provides a drastic (>40-fold) increase in cell yield of high quality, clinically relevant human dopaminergic neurons and significantly reduces the cost of making the cells for transplantation. Their method is so effective that the cell material from a 6-well plate is enough to generate material to treat 500 patients. With the help of large grants, this research group, in collaboration with BioLamina and other partners, aim to make the transition towards a Cell Therapy for Parkinson’s disease within a few years.
Efficient hepatocyte maturation and cell organization
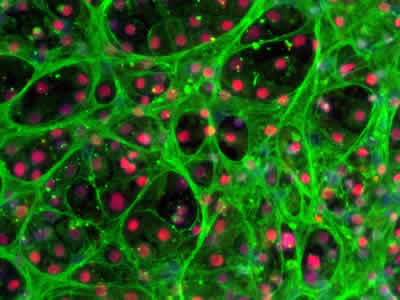
Stem cell-derived hepatocytes is a promising tool to generate more predictive in vitro toxicity models for preclinical safety evaluations and could become a more accessible, off the shelf alternative to organ donation. In November 2015, the group of Dr. David Hay at University of Edinburgh, Scotland, published a paper where they show a defined process to differentiate human ES cells towards hepatocytes with the use of a combination of laminin-521 and laminin-111 as culture substrates (Cameron et al., Stem Cell reports, 2015). Their results demonstrate efficient hepatocyte specification, polarized organization, enhanced maturation and significant improvement and stabilization of cell functions. Their results represent a significant advance and brings cell based hepatocyte therapies closer towards biomedical applications.
JoVE article for defined and scalable generation of hepatocyte-like cells from human pluripotent stem cells.
Maintained differentiation potential of satellite cell-derived myoblasts during long-term culture
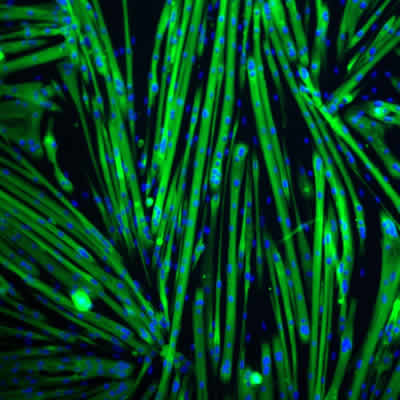
Large-scale expansion of myogenic progenitors is necessary to support the development of high-throughput cellular assays in vitro, and to advance genetic engineering approaches necessary to develop cellular therapies for rare muscle diseases. A recent publication from researchers at Icagen, Inc., USA, show that laminin-521 is a superior substrate for myogenic cell culture applications (Penton et al., Skeletal Muscle, 2016). Laminin-521 dramatically improves cell proliferation and maintains the differentiation potential of human satellite cell-derived myoblasts. Laminin-521 appears to increase differentiation potential without altering the traditional Pax7/MyoD paradigm and supports generation of larger myotubes and higher amounts of nuclei per myotube. Importantly, laminin-521 provides more consistent and reliable differentiation also over long-term culture expansion.
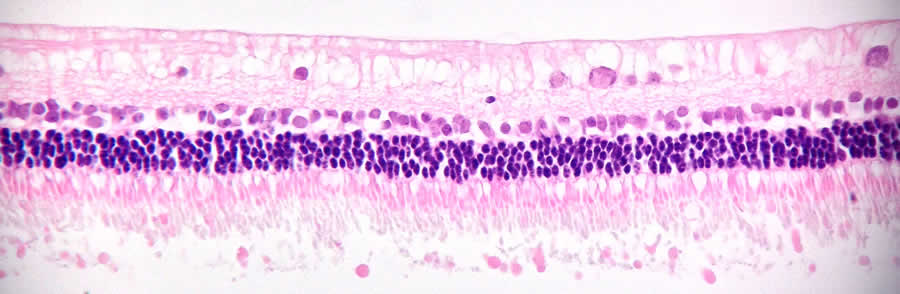
Clinically compliant hESC-derived retinal pigmented epithelial (RPE) cells
Age-related macular degeneration is the most common cause of severe vision loss in the Western world, with an economic burden on the health care system exceeding that of stroke. The single-layered RPE cell sheet is essential for normal neuroretinal and choroidal functions. In the beginning of 2016, the groups of Drs. Fredrik Lanner, Outi Hovatta and Anders Kvanta at Karolinska Institutet, Sweden, published a xeno-free and defined protocol for production of human ES cell-derived RPE cells on laminin-521 (Plaza Reyes et al., Stem Cell Reports, 2015). Suspension transplantations of the laminin-521 RPE cells into a large-eye rabbit model show efficient cell integration as a polarized, sub-retinal monolayer that were capable of rescuing overlying photoreceptors from induced damage. The work is currently ongoing to create new xeno-free GMP-grade human ES cell lines for the establishment of HLA-matched hESC/RPE bank, using BioLamina laminins for the translation into a clinical setting.
Read more about our different laminin cell applications
To conclude, by providing cells with their biorelevant matrix proteins, the natural, cellular interactions needed for cell adhesion, proliferation, organization and survival are stimulated. Derivation and expansion of hPSCs and succeeding cell specialization on specific laminin substrates could help bridge the gap between academic Stem Cell Research and the development of human Cell Therapy. To further facilitate the transition of these pre-clinical research protocols into clinical settings, BioLamina is currently developing a clinical grade product of laminin-521 (MX521). A clinical grade laminin-111 (MX111) is also under development to meet the needs for the dopaminergic neuron and hepatic Cell Therapy protocols. We believe that recombinant laminin culture substrates are one of the essential components for the unlocking of the great potential for stem cells, and that the research examples highlighted here will be followed by numerous more breakthrough papers for other applications. Please contact Scientific Support or visit our ScienceRoom for more data and publications based on human recombinant laminins.
Header Image: Human embryonic stem cells on laminin-521. Courtesy of Dr. Liselotte Antonsson, Karolinska Institute, Sweden
Footnotes
-
1. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521 based matrices under xeno-free and chemically defined conditions. Rodin et al., Nat Prot., 2014
-
2. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in de‑ ned and xeno-free environment. Rodin et al., Nat Commun., 2014
-
3. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Kirkeby et al., Cell Stem Cell, 2016
-
4. Recombinant Laminins Drive the Differentiation and Self-Organization of hESC-Derived Hepatocytes. Cameron et al., Stem Cell Reports, 2015
-
5. Laminin 521 maintains differentiation potential of mouse and human satellite cell-derived myoblasts during long-term culture expansion. Penton et al., Skeletal Muscle, 2016
-
6. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Plaza Reyes et al., Stem Cell Reports, 2015