Lonza’s Xcite® EV Technology: A Platform Approach for Producing Engineered Extracellular Vesicles
Extracellular vesicles (EVs) are being studied extensively as an innovative biological delivery modality and have emerged as a promising novel technology for carrying complex therapeutic payloads such as mRNA, DNA, or proteins. Exosomes are membrane-based extracellular vesicles with a diameter of 50–150 nanometers that are naturally released by cells within the body.
Exosomes play a key role in cell-to-cell communication, and therefore, influence nearby or distant targets. In addition to their ability to transfer cargo with precision, they have several advantages over other delivery vehicles such as lipid nanoparticles (LNPs) or viral vectors. For example, they may be able to support repeat dosing due to their broad tolerability and ability to evade the immune system response. Additionally, exosomes possess the ability to access biological barriers and deliver a wide range of cargo to specific target tissue types given their tropism profiles. These attributes make them favorable candidates to engineer as drug delivery systems.
Engineered extracellular vesicles to deliver clinically relevant payloads
While EVs may be exploited in their naïve form for their regenerative properties, efficient delivery of therapeutic cargo may be achieved using strategies to tether the payload onto the EV surface or package the payload into the lumen of EVs. This concept is demonstrated by Lonza’s engEx® exosome engineering technology, which was acquired by Lonza from Codiak Biosciences in 2023, and forms the basis of our Xcite® EV platform offering.
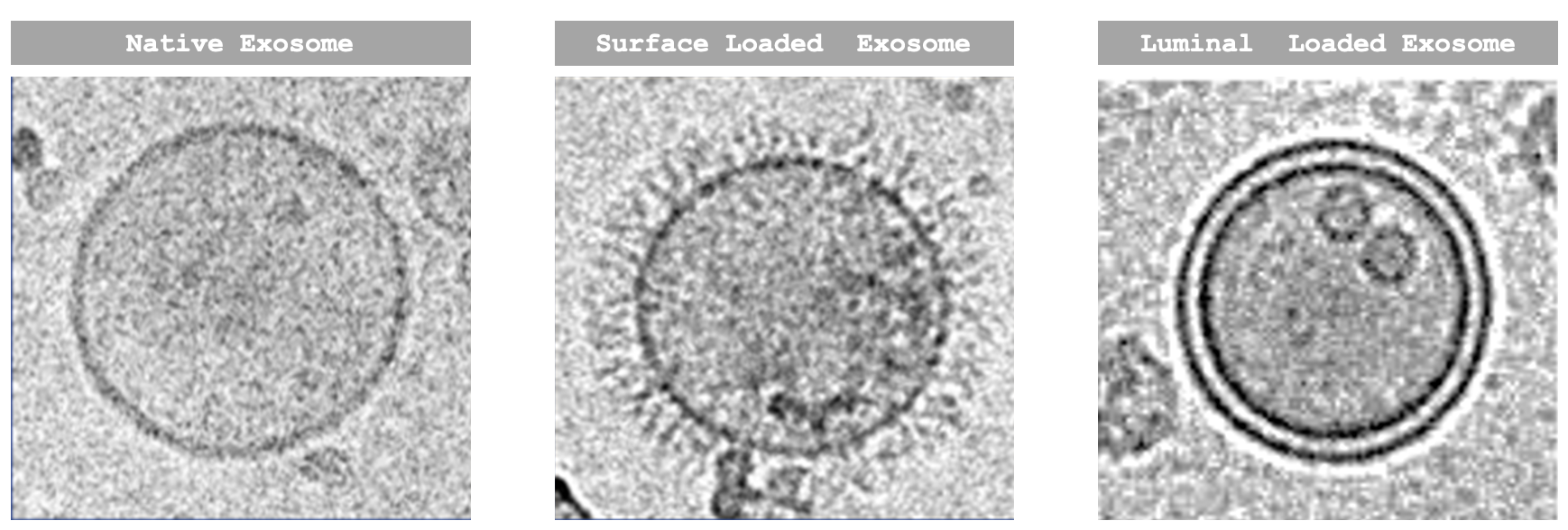
PTGFRN and BASP-1 are two scaffold proteins that have been identified to be over-expressed in EVs and associated with payload loading at high densities. To express therapeutic cargo, plasmids are engineered to have the expression target fused to one of the scaffold proteins – PTGFRN is used to tether the payload to the surface of the EV, while BASP-1 is used to load the payload into its lumen. The engineered plasmids are then transfected into a host cell line such as HEK 293 cells or mesenchymal stem cells (MSCs), resulting in the production of engineered exosomes expressing the therapeutic molecule. A vast variety of cargo (antibodies, enzymes, etc.) may potentially be expressed using this technology. Additionally, vaccine candidates may be developed similarly via the surface display of antigens on exosomes, triggering a sustained immune response 1.
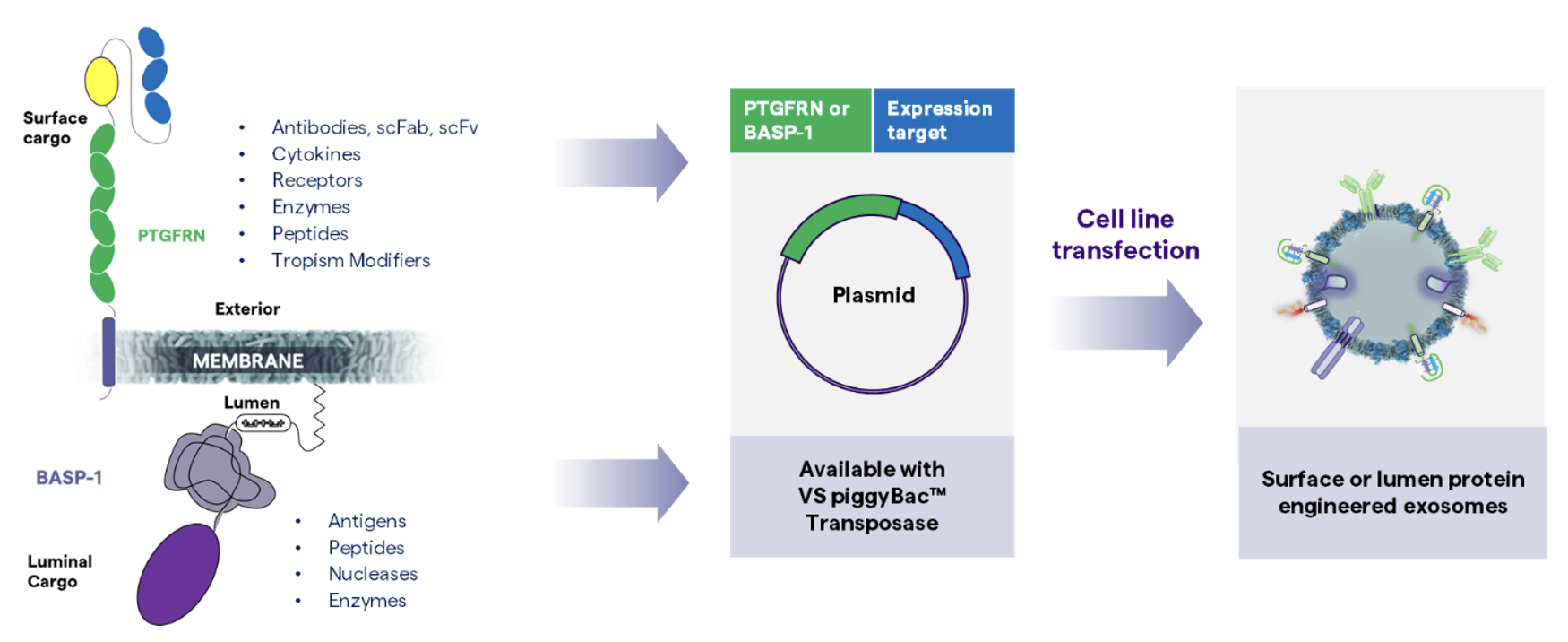
Furthermore, smaller molecules such as peptides or oligonucleotides may be linked to the surface of EVs by modification using Lonza’s proprietary linker technology, which may be performed following EV purification, avoiding the need for extensive cell engineering efforts.
Clinical applications of engineered extracellular vesicles
The engEx® technology has been tested to generate therapeutic candidates that have been validated in early clinical studies. Engineered exosomes targeting a wide range of disease exhibited excellent safety profiles and retained pharmacodynamic activity in a variety of animal models 2, 3. Drug molecules which typically promote systemic inflammation when delivered, such as IL-12 and cyclic dinucleotide STING agonists, have been shown to elicit no inflammatory response when delivered via engineered exosomes, enabling the potential to deliver larger doses for increased potency. Some examples of such therapies successfully evaluated in Phase 1 clinical trials include exoIL-12™ and exoSTING®, which have been used to treat T-cell lymphoma as well as other multiple refractory solid tumors 2, 3.
Engineered exosomes have also successfully been shown to inhibit challenging targets, such as exoASO-STAT6®, used to silence the activity of STAT-6 transcription factors for the treatment of hepatocellular carcinoma 4. This is achieved by engineering exosomes using linkers fused to the surface scaffold protein to selectively deliver antisense oligonucleotides (ASOs) targeting STAT-6 mRNA.
Licensing opportunities and partnerships
The Xcite® platform technology is available to license as part of our Lonza in Your Lab® approach. The license offer will provide you access to Lonza’s EV high producer clonal HEK 293 cell line and plasmids to generate engineered EVs. To learn more about beta testing opportunities and evaluating the Xcite® platform in your lab, please contact us at licensing@lonza.com or watch our webinar here for further information.
References
- Dooley, K. et al. (2021) “A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties”, Molecular Therapy, 29(5), pp. 1729–1743. doi:10.1016/j.ymthe.2021.01.020.
- Lewis, N.D. et al. (2021) “Exosome surface display of IL12 results in tumor-retained pharmacology with superior potency and limited systemic exposure compared with recombinant IL12”, Molecular Cancer Therapeutics, 20(3), pp. 523–534. doi:10.1158/1535-7163.mct-20-0484.
- Jang, S.C. et al. (2021) “ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance”, Communications Biology, 4(1). doi:10.1038/s42003-021-02004-5.
- Kamerkar, S. et al. (2022) “Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity”, Science Advances, 8(7). doi:10.1126/sciadv.abj7002.
Disclaimer
The information contained herein is believed to be correct. However, no warranty is made, either expressed or implied, regarding its accuracy or the results to be obtained from the use of such information. Lonza disclaims any liability for the use of this article and the use of the information contained herein is at your own risk. All trademarks belong to Lonza and are registered in Switzerland, and/or USA, EU, or belong to their respective third-party owners and are only being used for informational purposes. The products, methods, and processes described in this article may be covered by one or more granted patents or pending patent applications. For a complete listing please visit https://www.lonza.com/ip.
© 2025 Lonza. All rights reserved.