Media panels for AAV manufacturing: Finding the formulation for success
Gene therapies show great potential in the treatment of a variety of currently incurable genetic diseases. While viral vectors are the preferred method for gene delivery, their cGMP production has also created a bottleneck in the industry. The answer to this bottleneck is to increase viral vector titers and reduce operating footprint. For adeno-associated virus (AAV), this involves an industry move toward the use of suspension HEK293 cell lines.
One of the parameters influencing the efficiency and productivity of AAV manufacturing is the choice of a suitable cell culture medium. The medium used can have a profound impact on cell density and viral titer and needs to be carefully evaluated and selected with a specific workflow in mind.
However, there is no standardized approach when it comes to AAV manufacturing, and the ideal medium can be dependent on several factors including the cell line, vector optimizations, AAV serotype/pseudotype, and transfection parameters such as target cell density and transfection ratios. There are several barriers to improving viral yield and the process involved in optimizing media formulations to enhance titers can be time-consuming and expensive.
Utilizing media panels to select and optimize HEK293 suspension media for AAV production can accelerate the process and drive more gene therapies through clinical evaluation to commercialization. Media panels comprise a range of nutritionally diverse media formulations for manufacturers to test in their process with their cell line, expediting the selection process and allowing them to progress through media optimization much quicker. Media panels are revolutionizing the way AAV manufacturers establish optimized media for their vector manufacturing processes and present several benefits, which will be discussed below.
What makes the optimal medium?
There is no question that AAV manufacturers are looking to achieve the highest titers possible from their cultures. Higher titers allow facilities to make more vectors per batch—increasing productivity, reducing costs, and accelerating speed-to-market. Many manufacturers link their current low titers directly with the quality of their cell culture media, whether commercially sourced or manufactured in-house. Achieving higher productivity, while simultaneously maintaining consistent vector quality, is key for viral vector process developers.
When looking for a medium, factors such as batch-to-batch consistency should be considered. This is vital in securing overall product quality and meeting GMP standards for large–scale manufacturing.
In addition, manufacturers may wish to identify media enhancements that are platform driven and method independent. This allows media to be used for multiple products and can simplify the establishment of new processes, such as operating in perfusion, developing processes based on packaging or a stable producer cell line, or the manufacturing of alternative products in the future. Products such as media panels are designed to provide diversity in composition and to be easily customizable—allowing for increased flexibility.
Choosing a medium
Improving the efficiency of upstream AAV manufacturing processes is a top priority for gene therapy developers, and media appears to be one area where great improvement can be achieved. There are ultimately three available options when it comes to the selection of media for any process, and these are whether to opt for a catalog, “customized” catalog, or to develop a proprietary formulation. While there is no “one-size-fits-all” solution when selecting a medium, most manufacturers typically focus on catalog media, at least primarily, to use it as a “jumping off point” when developing an optimized medium.
The pace at which gene therapies are progressing through clinical stages, thanks to several support strategies put in place by regulatory agencies worldwide [1], means that an effective cGMP manufactured medium must be in place very early on in the process. For this reason, many developers prefer the more condensed development process associated with optimizing catalog media, which takes approximately 1–3 months compared to 1–2 years for a proprietary formulation.
The importance of developing the optimal medium is clear to manufacturers, however, they can find themselves facing several challenges when it comes to optimizing media formulations, regardless of whether a catalog or proprietary formulation is being used. The ideal choice of medium is entirely dependent on the specific process it is being used for. For this reason, manufacturers may spend months assessing and finalizing different formulations with little success. The disparity between the average reported titer and the average target titer for AAV manufacturers can be as much as two orders of magnitude.
Some of the challenges manufacturers may face in this endeavor include low titer and yield, low cell densities, and a cell culture medium seemingly lacking in consistency. These issues are further compounded by the time spent selecting and optimizing a medium, rising costs, and supply chain management challenges. As such, when sourcing a medium, the capabilities and capacity of the supplier are as important as the formulation itself.
Choosing a supplier
When choosing a media supplier, manufacturers—particularly those managing late–stage projects—place great importance on the security of the supply chain. They should seek a supplier that can maintain their supply of media once their product reaches the market. For earlier–stage projects operating at smaller scales, the focus might be more on the technical and regulatory support offered, with a requirement for information to support the set-up of their cultures, including assistance in doing this where necessary.
However, media and support requirements are specific to each project and should be evaluated when selecting a supplier to help ensure they are able to support customers throughout the AAV manufacturing journey. Media suppliers have a range of capabilities, including secure supply chains and regulatory support, among others, to streamline AAV manufacturing and speed-to-market, regardless of the stage or complexity of the project.
Media panels
Optimization of cell culture media is key for AAV manufacturers; however, when queried about how to achieve this goal, few are aware of any alternatives to in-house development. HEK293 cells are most commonly used when it comes to AAV manufacturing. While they can be cultured as either an adherent or suspension culture, the industry is moving away from adherent cultures due to challenges with scalability upon reaching clinical evaluation. This enhances the challenges associated with selecting and optimizing a medium, particularly when adapting cultures to suspension.
One option to speed up the optimization process is to use a panel medium. Media panels provide diverse, ready-to-use, non-GMP prototype formulations to screen against a variety of processes, but detailed protocols can also be provided to simplify experimental design. The increased likelihood of finding the ideal medium means reduced development times and costs associated with achieving higher titers.
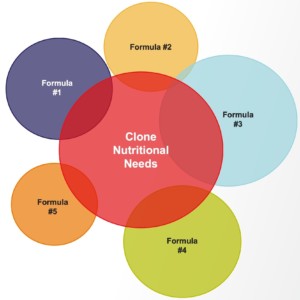
The formulations used in a viral vector HEK media panel are specially selected to provide a broad range of key nutrients to simplify the evaluation process. Testing a range of panel media with formulation diversity can lead to the identification of a final medium that provides better productivity and higher titers. Limited public formulation information is typically available with catalog media, making it challenging to set up truly diverse evaluations. The diversity provided by a well–designed media panel makes it simpler to identify key components driving productivity and quality. Each formulation is deliberately distinct from the others in the panel and may meet varying levels of the target population or clone’s nutritional needs (Figure 1). Utilizing the data from each formulation in the panel to identify key drivers can streamline the optimization process and support the development of an ideal formulation.
Using a media panel from an experienced and reliable supplier allows manufacturers to leverage their expertise to more easily select a formulation and optimize it successfully. Working closely with Field Application Scientists and a dedicated R&D team allows for the experienced interpretation of data collected when using the media panel and can result in a well-informed recommendation moving forward. This expertise can be used long after the media panel has been evaluated—for further analysis and optimization, rapid non-GMP prototyping, or full-scale GMP manufacturing.
The media within the panel should be scalable at a GMP-compliant manufacturing facility. This is because gene therapies move very quickly through regulatory processes, so a commercially viable workflow needs to be in place as soon as possible, including the medium. Knowing that the medium of choice can be manufactured at a GMP–compliant facility when an AAV-based gene therapy becomes commercialized can dramatically smooth the transition from clinical development to market. Manufacturers may also benefit from a media panel that is provided with regulatory support to streamline the move through clinical stages once an optimal medium has been selected.
How will media panels drive development?
The potential for media panels to change the way AAV-based gene therapy manufacturers select and optimize a cell culture medium is profound. The smaller sample sizes allow manufacturers to test a range of formulations and verify titers in-house. This can accelerate the development process by giving manufacturers relevant data about the impact of the medium on their process. They can then use this information to further optimize the medium if required and reach the optimal formulation much faster than starting from scratch. This capability, which has typically not been available to gene therapy manufacturers, will support the rapid progression of these therapies to the market.
About the Authors
 Céline Martin is currently Global Product Manager for Gibco Bioproduction. She has been part of Thermo Fisher Scientific since 2017 and joined to provide bioprocess scientists with the right tools to make the world healthier. She has a PhD in Bioprocessing from the French University of Lorraine focusing on the impact of bioreactor hydrodynamics on MSC microcarrier culture, and worked as an Upstream Development Scientist prior to joining Thermo Fisher Scientific optimizing CHO, HEK and ESC processes. Her current focus is on managing and developing new solutions for gene therapy manufacturing.
Céline Martin is currently Global Product Manager for Gibco Bioproduction. She has been part of Thermo Fisher Scientific since 2017 and joined to provide bioprocess scientists with the right tools to make the world healthier. She has a PhD in Bioprocessing from the French University of Lorraine focusing on the impact of bioreactor hydrodynamics on MSC microcarrier culture, and worked as an Upstream Development Scientist prior to joining Thermo Fisher Scientific optimizing CHO, HEK and ESC processes. Her current focus is on managing and developing new solutions for gene therapy manufacturing.

Jennifer Zatina is a Global Product Manager for Gibco™ PD-Express Services. She joined Thermo Fisher Scientific in 2020 and her areas of responsibility include Bioproduction Analytics and Media and Feed Panels. Prior to joining Thermo Fisher Scientific, she spent over 10 years working in product development and management in the dietary supplement industry.