Mitigating Risk beyond Xeno-Free: A Critical Component for Cell-Based Vaccines
The human serum albumin protein is known to perform a multitude of beneficial biological functions. From nutrient delivery to hungry cells, toxin and waste sequestration, and neutralization of free radicals, this unique ~66.5 kD protein does it all (Theodore Peters, Jr.’s All About Albumin). It has become widely accepted that most mammalian cell types require the presence of albumin in their growth environment in order to facilitate adequate in vitro expansion and predictable cellular function. Cells that have been utilized extensively for vaccine production, including VERO, Wi38, and MRC-5, are no exception. This two-part series will capture the usefulness of recombinant human serum albumin and its potential in all areas of the vaccine industry. First, we will show recombinant albumin’s ability to promote the expansion of cell lines used for the production of virus. Part two of this series will establish the utility of recombinant human serum albumin in virus particle stabilization.
Traditionally, vaccines produced by mammalian cells have involved cell culture protocols that require the cells be expanded in a fetal bovine serum-containing medium. While bovine serum does contain adequate amounts of albumin as well as a plethora of other helpful components that cells require for sufficient expansion and subsequent virus production, it also comes with the possibility of contamination, skyrocketing prices, and reduced availability. These caveats, as well as the recent advancements in serum free media formulations and increasing pressure from regulatory agencies, have helped transition the vaccine industry away from the use of bovine serum or bovine serum-derived components in all cell culture processes for novel emerging vaccines.
In order to create a xeno-free cell expansion medium, bovine serum and its intrinsic albumin source must be removed and replaced by human serum-derived albumin to maintain adequate cell expansion and proliferation prior to virus production. While this substitution is beneficial for cell growth and viability, the transition from bovine-sourced to human-sourced materials does not reduce the risk of potential viral contamination. As an industry, we are reminded from time to time of the reality of potential blood supply contamination as most recently demonstrated by Zika Virus. Confirming the absence of adventitious agents can be costly, and risk cannot be totally mitigated due to the failure to detect new or emerging virus types, which can become a real fiscal and scientific nightmare. A recombinant human serum albumin that exhibits equivalence to animal or human derived components would minimize viral contamination risks of vaccine products and at the same time maintain or improve vaccine productivity.
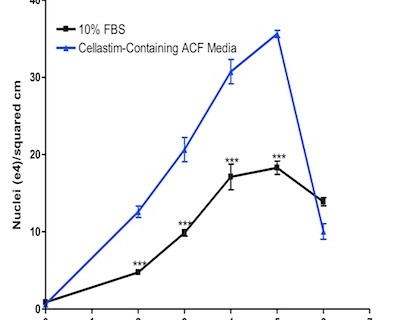
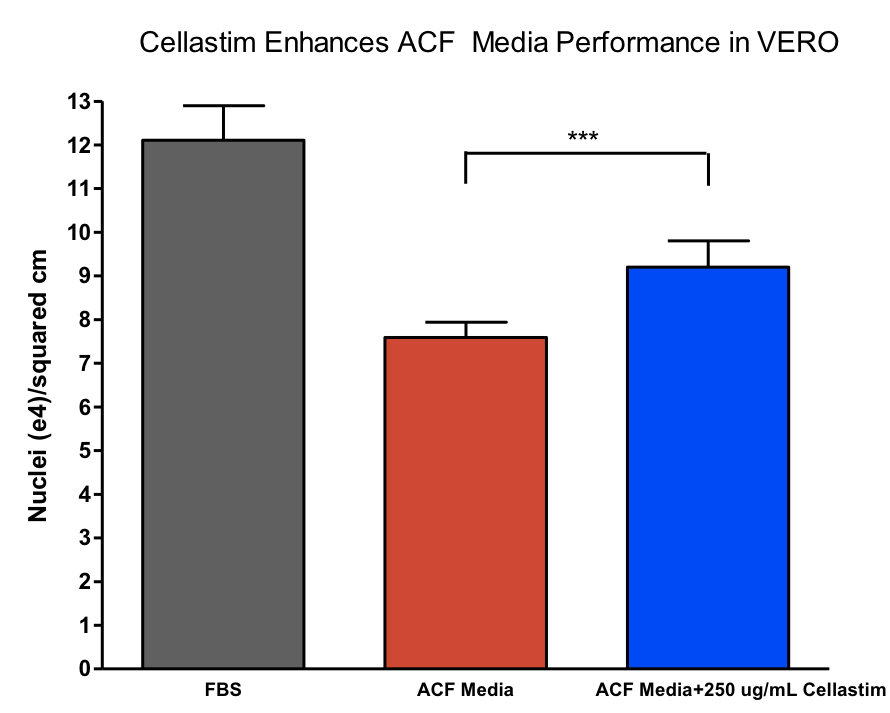
The recombinant albumin Cellastim has been shown to be capable of supporting a multitude of different cell types, including the vaccine workhorse VERO and the well characterized MRC-5 fibroblast line. The critical functionality of Cellastim is demonstrated in Figure 1 (below). Cellastim was supplemented into 13 different in-house animal component free (ACF) VERO media formulation candidates (i.e. media formulations completely void of any human or animal serum-derived components) and was shown to significantly boost VERO cell growth in all 13 formulations. The average performance of the 13 formulations with and without Cellastim supplementation are shown in Figure 1.
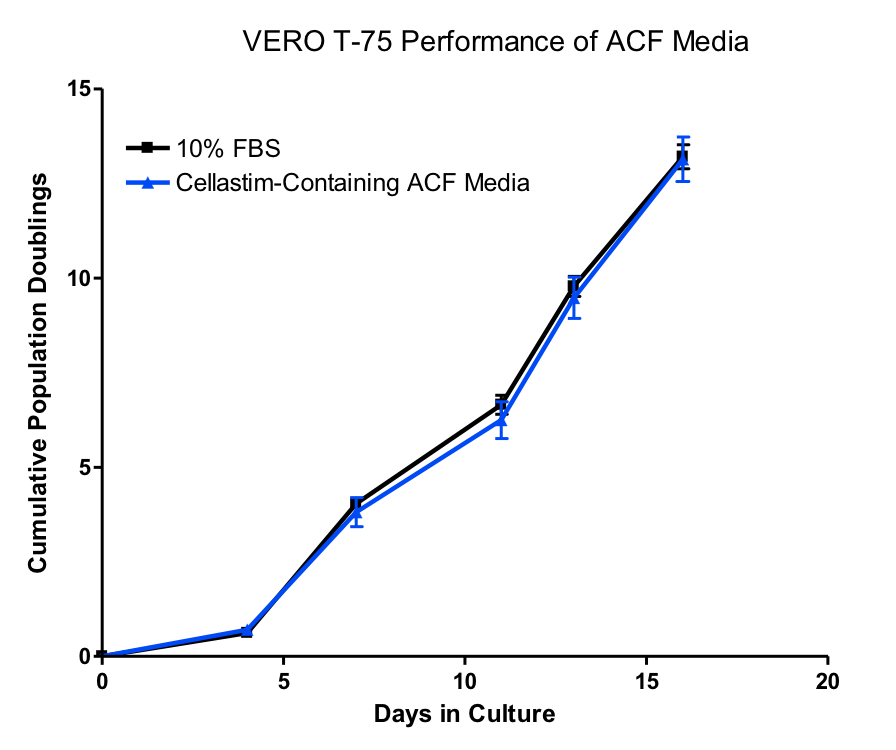
A single candidate formulation was selected to be further optimized, including further optimization of the Cellastim supplementation level, resulting in a serum free, animal component free, defined VERO medium able to propagate VERO cells for 5 passages comparable to FBS in T flasks (Figure 2).
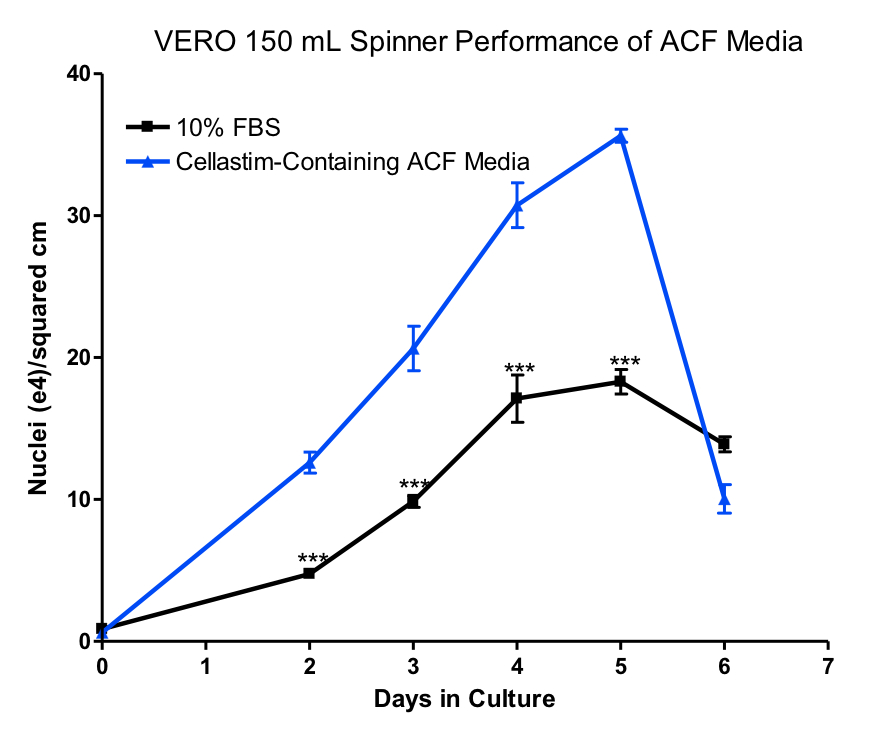
Scaling these cultures up to microcarrier culture on 150 mL spinner flasks (Figure 3), the Cellastim-containing ACF media resulted in significantly greater performance versus 10% FBS.
Similar results were obtained using another animal component free media for MRC-5 cells in 6-well plates (Figure 4):
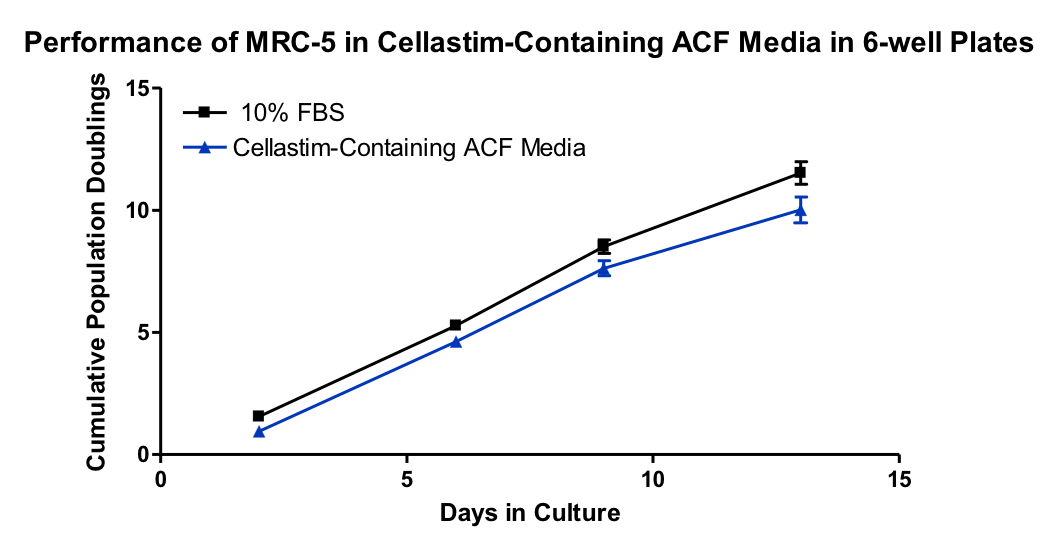
The combination of these results shows that Cellastim is an extremely useful component in the expansion of cells used to produce virus. Further, inclusion of recombinant animal-free human serum albumin provides risk mitigation of viral contamination in cell culture processes that won’t break the bank.
It is a common misconception that the cost of recombinant versions of critical proteins like human serum albumin is too high to be incorporated into large scale cell culture for vaccine production and other applications. With InVitria’s animal component free, recombinant HSA (Cellastim), the incorporation of a recombinant human albumin can be affordable and eliminate key disadvantages of working with an unpredictable product like human blood derived serum. Cellastim has already proven its unique ability to enhance performance with its recent incorporation into the production processes of several novel vaccines. While eliminating serum from a cell culture protocol is not always as simple as replacing the albumin source, InVitria offers a full product line of high performing, recombinant, animal-free proteins and supplements that are used to eliminate serum in cell culture from bench scale to large scale vaccine manufacturing. In addition, InVitria offers custom cell culture media development services that will ensure your animal component free media meets your requirements and will allow you less hassle, investment, and regulatory difficulty down the road. For more information on our product selection, visit www.invitria.com. To speak with one of our cell culture media development experts, e-mail CellCultureInfo@InVitria.com.