New ACF Medium to Expand and Differentiate Human Hematopoietic Cells into Erythroid Cells
Introduction
Red blood cells (RBCs) are the most abundant cell type in the blood. RBCs are continuously produced in the bone marrow as a result of the proliferation and differentiation of hematopoietic stem and progenitor cells (HSPCs). This process, called erythropoiesis, can be recapitulated in vitro by isolating HSPCs from umbilical cord blood (CB), bone marrow (BM) or peripheral blood (PB), and culturing them in an appropriate culture medium supplemented with various growth factors including the hormone erythropoietin (EPO). The ability to promote the proliferation and erythroid differentiation of HSPCs in culture has enabled research into the regulation of normal and aberrant erythropoiesis, and has opened the doors to the prospect of producing RBCs in vitro for transfusion of patients as a means to supplement the blood supply.¹ Culture-expanded erythroblasts are also excellent somatic target cells for generating induced pluripotent stem (iPS) cells by reprogramming.2,3
The Potential of In Vitro Expanded Erythroid Cells
Nowadays blood transfusions are very common and generally safe procedures that are life-saving for many patients. However, the shelf life of donated blood is limited and supplies are not always available when needed in large quantities or by patients with rare blood types. Although uncommon, blood transfusions can also cause severe adverse reactions in patients with disorders involving the abnormal production of hemoglobin, known as hemoglobinopathies, or for patients who have developed antibodies against blood group antigens after previous transfusions. Substituting natural blood with RBCs generated in vitro offers a potential solution to these problems.
Advances in the production of recombinant cytokines and cell culture media have enabled the development of culture methods that can yield thousands or even millions of highly enriched erythroblasts from small numbers of HSPCs in as little as 7 days. Studies have shown the potential application of cultured erythroblasts in transfusion medicine, as they have the potential to mature into reticulocytes and RBCs in vitro, and in vivo after infusion into mice or humans.4
Cultured erythroid progenitor cells are also an attractive starting population for the generation of iPS cells by reprogramming. Unlike other target cells, i.e. lymphocytes, cultured erythroblast progenitor cells do not possess genomic rearrangements and have proven to be very amenable to reprogramming. Large numbers of erythroid progenitor cells can be produced after only 7 days of culture, from HSPCs present in as little as 1 mL of adult donor peripheral blood. This is particularly important for the development of patient-specific iPS cell lines, a process which typically requires large numbers of somatic cells for reprogramming.
Challenges in Generating Erythroid Cells in Culture
Erythroid cell cultures are very robust and can yield thousands of erythroblasts after only 7-14 days of culture. However, it can be a challenge to obtain consistent cell yields between experiments due to variable quality and viability of HSPCs isolated from different donors. Protocols with standardized feeding and replating schedules can help decrease the variability of erythroid cell yields and avoid premature cell death due to overgrowth.
StemSpan™ Erythroid Expansion Medium (ACF) and Erythroid Expansion Supplement
StemSpan™ Erythroid Expansion Medium (ACF-E) is the newest member of the StemSpan™ family of hematopoietic expansion media and supplements from STEMCELL Technologies. StemSpan™ ACF-E is a new version of the original StemSpan™-ACF medium, that has been optimized specifically for use with the StemSpan™ Erythroid Expansion Supplement to drive the expansion and differentiation of human HSPCs into the erythroid lineage. Both StemSpan™-ACF and StemSpan™ ACF-E are recommended for applications where the use of a standardized, defined medium devoid of human- or animal-derived components is required. When combined with the appropriate expansion supplement, StemSpan™-ACF supports the robust expansion of CD34+ cells, myeloid cells and megakaryocytes. StemSpan™ ACF-E is recommended specifically for the expansion of erythroid cells in combination with the StemSpan™ Erythroid Expansion Supplement.
The StemSpan™ Erythroid Expansion Supplement contains an optimized combination of EPO, Stem Cell Factor (SCF) and Interleukin-3 (IL-3). By combining StemSpan™ ACF-E with the Erythroid Expansion Supplement, thousands of transferrin receptor (CD71) and Glycophorin A (CD235, GlyA) positive erythroblasts can be generated from small numbers of HSPCs isolated from CB (Figure 1), BM and PB after 7-14 days of culture in ACF conditions.
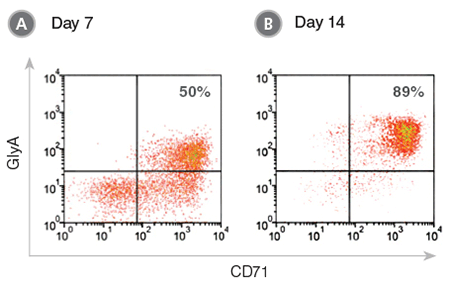
CB-derived CD34+ cells were cultured in StemSpan™ ACF-E medium containing Erythroid Expansion Supplement. After (A) 7 days and (B) 14 days, the cultured cells were stained with antibodies to CD71 and GlyA, and analyzed by flow cytometry. (A) During differentiation, the CD34+ cells progress to the CD71+GlyA+ erythroblast stage via CD71+GlyA– progenitors and pro-erythroblasts, which are still abundant on day 7. (B) By day 14 most cells have become CD71+GlyA+. A small population of cells may mature further in these cultures into CD71-/loGlyA+ normoblasts and reticulocytes.
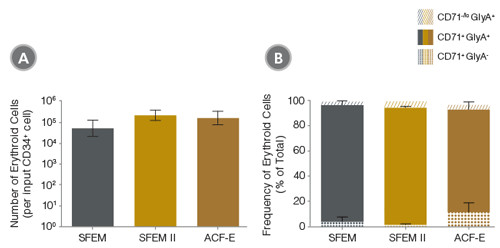
The average yield of erythroid cells expanded in StemSpan™ ACF-E is higher than in serum-free (but animal protein-containing) StemSpan™ SFEM medium, the medium of choice for numerous hematopoietic cell culture studies. Yields in ACF-E are also very close to those obtained in the optimized version of SFEM, StemSpan™ SFEM II (Figure 2).5,6 StemSpan™ ACF-E is thus a very robust medium for erythroid expansion even for applications where a medium free of animal- or human-derived components is not a strict requirement. StemSpan™ ACF-E is particularly useful for basic and preclinical studies directed toward the production of culture-expanded erythroid cells for transfusion, for which the availability of standardized animal-component free media will be critical. For more information see our website at www.stemcell.com or our new Technical Bulletin on StemSpan™ Media and Supplements for the Expansion and Differentiation of Erythroid Progenitor Cells.
If you have any questions please contact our Product Marketing Manager for StemSpan™ Media and Supplements, Sanja Sekulovic, at s.sekulovic@stemcell.com or visit our website at www.stemcell.com.
Footnotes
-
1. Rousseau GF et al. (2014) Large-scale production of red blood cells from stem cells: what are the technical challenges ahead? Biotechnol Journal 9(1):28-38.
-
2. Mack AA et al. (2011) Generation of Induced Pluripotent Stem Cells from CD34+ Cells Across Blood Drawn from Multiple Donors with Non-Integrating Episomal Vectors. PLOS One 6(11):e27956.
-
3. Chou BK et al. (2011) Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res 21(3):518-29.
-
4. Giarratana MC et al. (2011) Proof of principle for transfusion of in vitro-generated red blood cells. Blood 118(19):5071-9.
-
5. Leberbauer C et al. (2004) Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. 105(1):85-94.
-
6. Delaney C et al. (2010) Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med.16(2):232-6.